The American Cancer Society’s Oncology in Practice: clinical management (2018)
Edited by American Cancer Society
Введение
Гастроинтестинальные стромальные опухоли (GIST) являются наиболее распространенным вариантом сарком и составляют 80% желудочно-кишечных мезенхимальных новообразований, хотя по-прежнему считаются редкими новообразованиями, представляя всего 0,1-3% всех злокачественных опухолей желудочно-кишечного тракта. Предполагается, что они возникают из интерстициальных клеток Кахала (Cajal), которые являются компонентами автономной нервной системы кишечника и служат в качестве интестинальных пейсмейкеров [1]. Вследствие неопределенности в ранних методах диагностики GIST часто ошибочно диагностировались как кишечные лейомиомы, лейомиосаркомы, лейомиобластомы, шванномы, опухоли гастроинтенстинальных автономных нервов или другие сходные гистологически опухоли мягких тканей [2].
Однако два ключевых события за последние 15 лет улучшили понимание и лечение GIST: (i) идентификация конститутивно активных сигналов (онкогенная мутация c-KIT и PDGFRA генов, кодирующих рецепторные тирозинкиназы) [3] и (ii) создание ингибиторов тирозинкиназ (TKI), которые подавляют рост опухоли путем специфического таргетинга и ингибирования этого сигнала [4]. Появление эффективной терапии драматически улучшило исходы пациентов с GIST, тем самым пересмотрев роль хирургического вмешательства. Более того, развитие менеджмента GIST стало доказательством принципа трансляционной терапии в онкологии, подтверждая, что специфическое ингибирование активности опухоль-ассоциированной рецепторной тирозинкиназы может быть эффективным лечением рака.
Инцидент
Самые последние данные Национальной Программы по Надзору, Эпидемиологии и Конечным Результатам Национального Института Рака (SEER) показали почти удвоение распространенности всех мезенхимальных опухолей GI (более 80% для GIST), что, вероятно, связано с повышением идентификации и распознавания [5]. В настоящее время заболеваемость в Соединенных Штатах оценивается примерно в 5000 новых случаев в год [6]. Международные популяция-базисные исследования оценивают годовой показатель инцидента в пределах от 10 до 15 случаев на миллион населения, а показатель распространенности примерно 129 человек на миллион [7,8].
Этиология и факторы риска
Для GIST отсутствуют расовые, этнические или гендерные предпочтения. Медианный возраст на момент диагноза составляет 60 лет. GIST редко встречаются у детей, за исключением случаев семейного синдрома или триады Карни (Carney) [9]. В отличие от взрослых дети (i), как правило, имеют мультифокальные GIST желудка, (ii) их новообразования являются диким типом для KIT и PDGFRA, и (iii) их опухоли имеют более высокий инцидент метастазов в лимфатические узлы [10].
Наследственные GIST
Подавляющее большинство GIST являются спорадическими, хотя сообщено о семейных когортах с герминативными KIT или PDFGRA мутациями. Основные различия между индивидуумами с GIST, вторичными по отношению к зародышевой KIT мутациям по сравнению со спорадическими GIST, включают более молодой возраст и мультифокальную болезнь на момент диагноза и редкую метастатическую диссеминацию [11]. Фенотип родственных GIST популяций с зародышевыми KIT мутациями включает гиперпигментацию кожи и диффузную гиперплазию интестиального myenteric сплетения. Сообщалось также о случаях ассоциированных меланомы, рака молочной железы и пищевода [12].
Гастральные GIST могут быть компонентом триады Карни (Carney)и синдрома Карни-Стратакиса (Carney–Stratakis), и часто демонстрируют медленное, индолентное течение. Триада Карни включает GIST желудка (ранее считавшейся лейомиосаркомой), функциональные вненадпочечниковые параганглиомы и хондромы легких [13]. Лейомиомы пищевода и адреномы коры надпочечников были недавно добавлены в качестве компонентов синдрома. ~80% диагностируются до 30 лет и ~ 85% наблюдаются у женщин. GIST, выявленные у пациентов с триадой Карни, не имеют соматических KIT или PDGFRA мутаций. Синдром Карни-Стратакиса описывает семейные случаи GIST и параганглиом [14]. Мутации в генах, кодирующих несколько субъединиц сукцинатдегидрогеназы, найдены у родственников с синдромом Карни-Стратакиса [15].
Approximately 7% of individuals with the autosomal dominant disorder von Recklinghausen’s neurofibromatosis (NF1) have GIST. Contrary to the equal sex distribution noted for type 1 and/or atypical NF1 deletions, these NF1-associated GISTs have a higher incidence in women (1.4:1), and are most commonly manifested by a multifocal presence in the small intestine [16]. While these individuals express KIT and PDGFRA point mutations in 8% and 6% of their GISTs respectively, NF1 mutations have not been identified in non-NF1 individuals with sporadic GISTs [17–19].
Клиническая картина
Первичные опухоли
In one early study, 69% of GISTs were symptomatic, 21% were discovered incidentally at surgery, and 10% were discovered at autopsy [20]. GISTs commonly arise in the stomach (50–70%), small intestine (25–35%), colon and rectum (5–10%), mesentery or omentum (7%), and esophagus (<5%), but may occasionally arise in the duodenal ampulla, appendix, gallbladder, and urinary bladder. GISTs are often friable and highly vascular, and commonly present with bleeding. They may cause life-threatening hemorrhage by erosion into the bowel lumen [21]. Alternatively, intestinal obstruction may lead to frank perforation or tumor rupture resulting in potentially catastrophic intraperitoneal bleeding and/or dissemination by peritoneal seeding. Smaller tumors often remain asymptomatic, only incidentally detected on radiographic studies, during endoscopy or at laparotomy.
Метастатическая болезнь
Presentation with metastatic disease occurs in approximately 15–50% of patients with GIST. Common sites include the liver, peritoneum, and omentum, while metastases to lymph nodes and extra-abdominal structures (brain, bone, lung, and subcutaneous tissues) occurs in <5% of patients. Extra-abdominal metastases are usually only noted later in the course of the disease [22].
Диагноз
Радиографические обследования
Contrast-enhanced computed tomography (CT) of the abdomen and pelvis is the initial imaging study of choice for a suspected or confirmed GIST. Primary GISTs typically appear as well-circumscribed masses within the walls of hollow viscera on CT (Figure 11.1(a)), while magnetic resonance imaging (MRI) may help characterize metastatic liver or primary perirectal disease (Figure 11.1(b)) [23]. Although [18F]fluoro-2deoxy-D-glucose positron emission tomography (FDG-PET) may help characterize masses ambiguous on CT, monitor response to therapy, and detect emergence of drug-resistant clones for most patients with suspected neoplasms, it is not de rigueur for the routine management of patients with primary advanced disease. Recommendations concerning the utility of PET in patients with metastatic GIST have been equivocal. Consequently, they may be used sparingly.
Эндоскопия, тонкоигольная аспирация и биопсия
The endoscopic appearance of a primary GIST is typically a submucosal lesion often indistinguishable from GI leiomyomas. GIST may be located in either the upper or lower GI tract [24] and ulceration may or may not be present. Upper endoscopy with endoscopic biopsy has a low diagnostic yield (17–42%), and endoscopic ultrasound alone has not been found necessary to evaluate a confirmed GIST. Conversely, endoscopic ultrasound-guided fine-needle aspiration (FNA) has a diagnostic yield and sensitivity nearing 80%. Immunohistochemistry [25] or reverse-transcriptase polymerase chain reaction analysis of FNA specimens for KIT mutations may be required to confirm a diagnosis definitively.
Preoperative biopsy is not routinely necessary for a primary, resectable mass suspicious for GIST. In theory, biopsy may rupture a suspected GIST, increasing the risk of dissemination. However, biopsy may be appropriate if neoadjuvant therapy is a consideration, if the differential diagnosis includes entities such as lymphoma that would dictate a different treatment, or if there is metastatic disease.
Гистопатология и молекулярная патология
In 1983, Mazur and Clark proposed the term “GIST” to describe “intraabdominal nonepithelial neoplasms that lacked the ultrastructural features of smooth muscle cells and the immunohistochemical characteristics of Schwann cells” [26]. GISTs are classified histologically into one of three categories: epithelioid type, spindle cell type, or mixed type. While most gastric GISTs feature an epithelioid phenotype, esophageal, small intestinal, colonic, and anorectal tumors more often feature spindle cell morphology.
Hirota et al. reported two critical findings in 1998 [3]: (i) near-universal expression of the transmembrane receptor tyrosine kinase KIT in GIST, and (ii) gain-of-function mutations in the corresponding c-KIT proto-oncogene. Normally activated by binding Kit ligand, also known as “steel factor” or “stem cell factor”, the KIT receptor plays a critical role in hematopoiesis, and gametogenesis, and the maintenance of intestinal pacemaker cells.
More than 85% of GISTs have activating KIT mutations, and mutated Kit remains constitutively active even in the absence of ligand binding resulting in both unregulated cell growth and malignant transformation [27]. These mutations commonly occur in exon 11 (57– 95% of cases), exon 9 (10–18%), and exons 13 and 17 (both 1–4% respectively) [28–30]. GISTs with KIT exon 9 mutations predominantly arise in the small intestine, and homozygous mutant GISTs are often associated with metastatic disease. Mast/stem cell growth factor receptor (SCFR), also known as tyrosine-protein kinase Kit, proto-oncogene cKit, or CD117, is a protein that is encoded by the KIT gene in humans [31]. The presence of CD117 immunoreactivity does not necessarily correlate with a KIT gene mutation, and conversely a KIT mutation can exist in the absence of CD117 immunoreactivity. Nevertheless, because CD117 expression (KIT-positivity) is characteristic of most GISTs, and is not characteristic of other gastrointestinal smooth muscle tumors such as leiomyosarcoma, GISTs are now identified by immunohistochemical staining for this antigen, a practice that has enhanced the understanding of the prevalence of this disease [32].
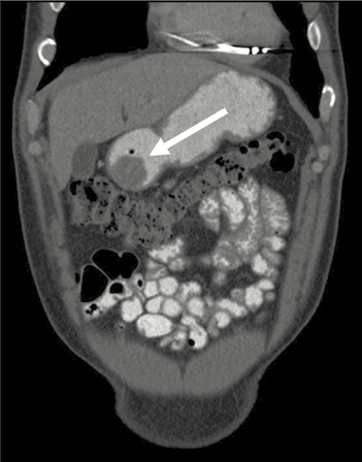
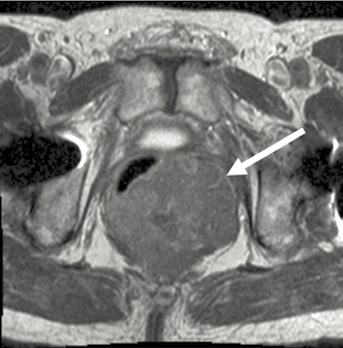
Figure 11.1. Cross-sectional imaging depicting (a) a computed tomography image of a distal gastric GIST (arrow) and (b) a magnetic resonance image of a nearly obstructing rectal GIST (arrow).
Activating mutations in a gene encoding a related receptor tyrosine kinase, platelet-derived growth factor receptor alpha (PDGFRA), have been identified in approximately 35% of GISTs lacking KIT mutations [9]. PDGFRA mutations in GISTs have been identified in exon 18 (2–6%), exon 12 (1–2%), and in exon 14 (<1% of the time) [9,10,33]. No differences in the activation of downstream signaling intermediates have been observed between PDGFRA-mutant and KIT-mutant tumors, suggesting that mutant PDGFRA provides oncogenic signals that parallel those of mutant KIT.
Wild-type GISTs exhibit no detectable KIT or PDGFRA mutations and utilize other pathways for pathogenesis [34]. Overexpression of insulin-like growth factor-1 receptor in addition to several reported mutations of succinate dehydrogenase subunits and BRAF have also been identified in a small percentage of wild-type tumors [35–37].
Прогностические факторы
While tumors under 1 cm are likely have a low risk of recurrence, no tumors can be definitively called benign. The three established, independently prognostic factors are tumor site of origin, tumor size (single largest dimension), and mitotic index (the most important and strongest predictor of recurrence) (Figure 11.1) [38]. Small bowel GISTs have a higher risk of progression than gastric GISTs of comparable size and mitotic count with jejunal and ileal tumors having the highest rate of metastasis followed by duodenal and rectal tumors respectively (Table 11.1).
A validated nomogram [39] assigns points for each of these three prognostic factors. The probability of remaining recurrence-free at 2 and 5 years can then be predicted by using the point totals in a summative manner (Figure 11.2). It is important to note that the nomogram bases the probability of recurrence on a cohort of patients diagnosed in the pretyrosine kinase inhibitor (TKI) therapy era.
Point mutations can also affect prognosis, and location is important. Insertions of KIT exon 11 appear to have a favorable prognosis, while deletions involving amino acid W557 and/or K558 have a poor prognosis [40]. Patients with PDGFRa exon 18 D842V mutations are resistant to imatinib therapy, whereas those with mutations in exon 12 are responsive to imatinib [12]. Additional adverse prognostic factors observed in some but not all studies included KIT exon 9 mutations, aneuploidy, high cellular proliferation index, and telomerase expression.
Стадийность
The most common system used for the staging of GISTs is the TNM system of the American Joint Committee on Cancer (AJCC) [41]. Once the T, N, and M categories have been assigned, the mitotic rate in combination with TNM classification is combined with the starting tumor location to predict prognosis. Hence, GISTs that start in the stomach or omentum are classified in one group, while GISTs of the esophagus, small intestine, colon, rectum, and/or peritoneum are separately defined. Tumors are finally described as resectable or unresectable depending on size, location, spread, and the ability of the patient to sustain an operative resection. Tumors may be defined as marginally resectable if complete removal is uncertain.
Таблица 11.1. Оценка риска для первичных гастроинтестинальных стромальных опухолей. Риск рецидива основан на данных из доиматинибной эры.
| Частота митозов | Размер опухоли (см) | Пациенты с прогрессирующей болезнью/классификация рисков, основанная на области происхождения (%) | |||
| Желудок | 12-перстная кишка | Тощая/подвздошная кишка | Прямая кишка | ||
| ≤5 per 50 HPF | ≤2 | 0 | 0 | 0 | 0 |
| >2, ≤5 | 1.9/очень низкий | 8.3/низкий | 4.3/низкий | 8.5/низкий | |
| >5, ≤10 | 3.6/низкий | –* | 24/умеренный | –* | |
| >10 | 12/умеренный | 34/высокий | 52/высокий | 57/высокий | |
| >5 per 50 HPF | ≤2 | –* | –* | –* | 54/высокий |
| >2, ≤5 | 16/умеренный | 50/высокий | 73/высокий | 52/высокий | |
| >5, ≤10 | 55/высокий | –* | 85/высокий | –* | |
| >10 | 86/высокий | 86/высокий | 90/высокий | 71/высокий | |
HPF, high-power field; *,insufficient data.

Рисунок 11.2. Нормограмма для предсказания вероятности 2-летней и 5-летней безрецидивной выживаемости. Points are assigned for size, mitotic index, and site of origin by drawing a line upward from the corresponding values to the “points” line. The sum of these three points, plotted on the “total points” line, corresponds to predictions of 2-year and 5-year recurrence-free survival (RFS). HPF, high-power field.
Терапия первичной болезни
Предоперационная терапия первичной болезни
The identification of effective, relatively well-tolerated, orally available TKIs including imatinib mesylate, sunitinib malate, and regorafenib have dramatically changed treatment practices. Imatinib selectively inhibits several tyrosine kinases, including KIT, PDGFRA, and BCR-ABL. Up to 80% of patients with metastatic GIST show a radiographic partial or complete response on imatinib or demonstrate stable disease. This success prompted investigation of its use in the perioperative period given the high incidence of recurrence often seen following resection of primary, nonmetastatic GIST [42–46].
In the Radiation Therapy Oncology Group (RTOG) 0312 phase II trial [43,47] patients with primary GIST measuring ≥5 cm (group A) or resectable recurrent/metastatic GIST measuring ≥2 cm (group B) received neoadjuvant imatinib (600 mg/day) for 8–12 weeks prior to surgical intervention, followed by maintenance postoperative imatinib for a duration of 2 years. Estimated 5-year progression-free survival (PFS) was 57% in group A and 30% in group B while 5-year overall survival (OS) was 77% in group A and 68% in group B, respectively. With regards to disease-specific survival, in group A, seven of 11 patients experienced disease progression >2 years from registration, and six of seven of the patients with progression had stopped imatinib before progression. In group B, disease progressed in 10 of 13 patients >2 years from registration, and six of 10 patients with progression had stopped imatinib before progression. This first multi-institutional neoadjuvant study confirmed the use of neoadjuvant imatinib as a safe practice. The authors also suggested considering longer treatment durations in intermediate-tohigh-risk GIST patients receiving adjuvant therapy especially given that a high percentage of patients experienced disease progression after discontinuation of maintenance imatinib following surgery.
In a single-institution study of neoadjuvant imatinib administered for 3, 5, or 7 days prior to surgery (followed by 2 years of postoperative imatinib), tumors resected from individuals treated with preoperative imatinib demonstrated increased tumor apoptosis compared to treatment-naпve patients [47].
The German APOLLON study [48] was a prospective, open label, phase II study evaluating the effects of neoadjuvant treatmentonpatientswithlocallyadvancedKIT-orPDGFRA-positive GISTs. Enrolled patients received imatinib daily for 6 months, in the absence of disease progression or unacceptable toxicity. At 8 weeks, patients underwent FDG-PET examination to assess response in parallel to CT imaging. Unlike other trials, patients did not receive adjuvant treatment postoperatively as this was a purely neoadjuvant study. R0 resections were performed in 30/34 patients, and two patients showed M1 disease at resection. This study concluded that neoadjuvant treatment with imatinib for 6 months was not only a safe treatment in patients with locally advanced disease, but also that the extent of the operation could be significantly downstaged following pretreatment.
Although many studies have documented that imatinib significantly improves the outcomes of most patients with advanced GIST (as discussed later), demonstrating its utility in the neoadjuvant setting is challenging, and there are still unanswered questions. Defining a duration of treatment for those that are responsive would be helpful. Furthermore, it is unclear what long-term survival benefits there are to neoadjuvant imatinib therapy.
Though it is still unclear when precisely to use neoadjuvant imatinib in practice, situations where tumor shrinkage potentially allows the scope of an operation to be downstaged or simplified offer good indications for its use, as evidenced by the APOLLON study, and illustrated in Figure 11.3. In the case study demonstrated in Figure 11.3, pretreatment and posttreatment/preoperative axial MRI images of a patient with a rectal GIST treated with neoadjuvant imatinib showing dramatic shrinkage of tumor. The patient initially presented with partially obstructive symptoms, but due to rapid improvement of symptoms on imatinib, he was able to avoid a diverting colostomy. After completion of 7 months of neoadjuvant imatinib, he underwent a transanal full thickness rectal wall resection of his GIST with excellent preservation of sphincter function. Alternatively, shrinking a tumor to permit laparoscopic as opposed to open resection, even if the extent of resection is unlikely to change, may be another indication for neoadjuvant imatinib.
Хирургия
Техника
Regardless of whether neoadjuvant therapy is used, the standard of care and only potentially curative therapy for patients with primary, localized, and resectable GISTs is surgical intervention [49,50]. The abdomen should be thoroughly explored at laparotomy to identify and remove any previously undetected metastatic peritoneal deposits (although if metastatic disease is known preoperatively, systemic therapy with imatinib should be first-line therapy, not surgery). GISTs generally do not invade other organs beyond the site of origin despite CT appearance, although primary tumors may demonstrate inflammatory adhesions to surrounding organs. In a series of 140 patients with primary gastric GISTs, wedge resections were performed in 68%, partial gastrectomies in 28%, and total gastrectomies in only 4% [51]. In cases where the tumor size is quite large and invasive into surrounding critical organs, a more extensive resection (total gastrectomy for a large proximal gastric GIST, pancreaticoduodenectomy for a periampullary GIST, or abdominoperineal resection for a low rectal GIST) may be necessary. However, several recent multi-institutional retrospective series have questioned the need for these resections given that neoadjuvant imatinib may downstage the scope of the operation. In adult patients, lymphadenectomy is not required since lymph nodes are rarely involved, while lymphadenectomy in pediatric patients is generally considered on a case by case basis given their higher frequency of nodal involvement [52,53].
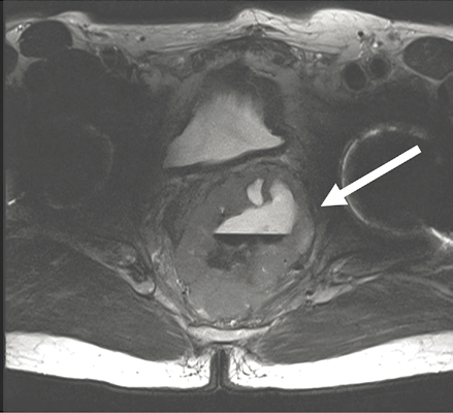
Pretreatment T0
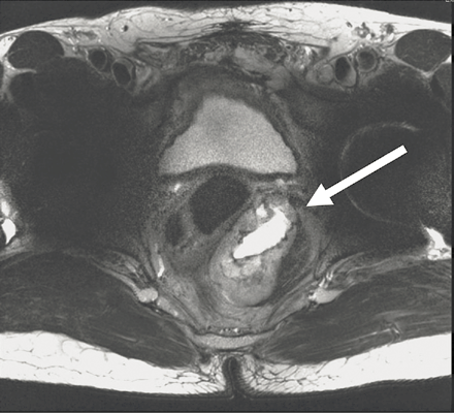
Post-treatment, preoperative, T7 months
Figure 11.3. Pretreatment (a) and post-treatment (b) axial magnetic resonance images of a patient with a rectal GIST treated with neoadjuvant imatinib. Patient initially presented with partial obstructive symptoms, but due to rapid improvement of symptoms on imatinib, he did not require a diverting colostomy. After 8 months of neoadjuvant imatinib, he underwent a transanal full thickness rectal wall resection of his GIST with excellent preservation of sphincter function.
The goal of the operation should be an R0 (no residual gross or microscopic disease) resection. Multiple studies have shown that a macroscopically complete resection with negative or positive microscopic margins (R0 or R1 resection, respectively) is associated with better prognosis than a macroscopically incomplete resection (R2 resection). Violation of the tumor capsule or tumor rupture during surgery is associated with an increased risk of recurrence (virtually that of metastatic disease), and should therefore be avoided. Although the optimal margin of resection is still unclear, post hoc analysis of a randomized trial evaluating the utility of adjuvant imatinib mesylate therapy for 1 year after resection of primary GISTs at least 3 cm in size demonstrated that regardless of the use of adjuvant imatinib, there was no significant difference in recurrence-free survival (RFS) for patients undergoing an R0 versus R1 resection [54]. There are no data indicating that patients who have an R1 resection require re-excision, and lack of difference in RFS between patients undergoing R0 versus R1 resection suggests that re-excision may be avoided. However, given the fact that margins may retract after resection, or the pathologist may trim the staple line (converting a technically negative microscopic margin into a positive one), all cases of positive microscopic margins should be carefully reviewed by a multidisciplinary team of pathologists and surgical and medical oncologists to assess the need for re-excision. The impact of this intervention on OS still remains unknown.
All GISTs 2 cm in size or greater should be resected when possible, as none of these can be considered benign; however, the natural history of GISTs under 2 cm in size is unclear, and their management is more debatable. While the low risk of progression of GISTs under 2 cm may support a more conservative approach, an accurate mitotic index cannot be determined by biopsy or FNA and therefore observation for these tumors is difficult to recommend. As such, resection of GIST measuring 1–2 cm should be considered, and the risks and benefits of surgery versus observation should be reviewed with the patient. Given the higher risk of aggressive behavior of small bowel and colon GISTs, any tumor in these locations should be resected irrespective of size. Conversely, multiple studies have established that subcentimeter gastric GISTs are relatively common, were detected in 10–35% of patients undergoing resection for other gastrointestinal malignancies [55–57] and were detected in approximately 23% of autopsies in adults over the age of 50 [58]. Despite their relative frequency, few of these neoplasms appear to become clinically relevant and most gastric GISTs under 1 cm in size may be followed. Until further data are available, the most appropriate management of such small tumors remains uncertain.
Although endoscopic resection of small gastric GISTs has been reported, this cannot be recommended. Because GISTs involve the muscularis propria, attempts at endoscopic resection risk leaving gross tumor behind and could result in perforation due to the depth of the lesion. All small GISTs that are symptomatic (e.g., hemorrhage from erosion through the mucosa) or increase in size on serial follow-up should be resected, regardless of their size.
Two early studies confirmed both the safety and feasibility of a laparoscopic approach when resecting GISTs, and laparoscopic or laparoscopy-assisted resection of primary GISTs may be performed using standard oncologic principles [59,60]. More recent studies [61,62]. demonstrated that compared with open resection, laparoscopic resection of gastric GISTs offers the advantages of less trauma, faster recovery, less blood loss, and shorter hospital stay. Furthermore, laparoscopic resection of gastric GIST can be performed more safely, more effectively, and with faster postoperative recovery using a gasless technique when compared to a standard open technique [63].
Послеоперационная терапия первичной болезни
Several prospective trials have explored the role of adjuvant therapy with imatinib combined with resection of primary disease (Table 11.2) [49,64–69]. In the phase III ACOSOG Z9001 trial [66], patients were randomized to receive either placebo or imatinib postoperatively for 12 months following complete resection of primary GISTs at least 3 cm in size. Interim analysis confirmed that the 1-year RFS was significantly better in the imatinib arm (97% vs 83%, P= 0.0000014), and the trial was halted early. Interestingly, once recurrences were observed, the slopes of the Kaplan–Meier curves representing the two treatment arms were similar. This suggested that 1 year of adjuvant imatinib may delay recurrence of primary GIST, but may not necessarily cure patients during this short follow-up interval. Furthermore, there was no difference observed in OS between the two treatment arms.
In the phase III SSG XVIII trial [44], patients with high risk or more clinically advanced GISTs were randomized to receive imatinib postoperatively for 12 months versus 36 months following complete resection. This trial confirmed that patients treated with 36 months of adjuvant imatinib had longer 5-year RFS than those treated with 12 months of adjuvant imatinib (66% vs 48%, P <0.01). More importantly, it demonstrated that patients treated with a longer duration of imatinib had an improved 5-year OS (92% vs 82%, P= 0.02).
Casali et al. conducted an open-label randomized trial evaluating adjuvant imatinib for 2 years in localized, surgically resected, high/intermediate risk GIST [45]. Specifically, patients were randomized between 2 years of postoperative imatinib (400 mg daily) and no postoperative treatment. With a median follow-up of 4.7 years, there was no statistically significant difference in imatinib-free survival though there was a trend in favor of imatinib when the analysis was limited to patients at high risk of recurrence. Imatinib did confer improved RFS at 3 years and at 5 years (P <0.001), confirming other studies. Fiveyear OS was similar.
The PERSIST-5 trial (5 years of adjuvant imatinib) [69] has completed accrual. Based on the available data, the current standard of care is that patients with intermediateto high-risk GIST should be treated with a minimum of 36 months of adjuvant imatinib. Any adjuvant therapy of 1 year’s duration or longer improves RFS, but improvement in OS is not seen until completion of 3 years of therapy.
Table 11.2. Multi-institutional trials of the use of neoadjuvant or adjuvant imatinib in the perioperative management of resected primary gastrointestinal stromal tumors.

ACOSOG, American College of Surgeons Oncology Group; EORTC, European Organisation for Research and Treatment of Cancer; HPF, high power fields; RTOG, Radiation Therapy Oncology Group; RFS, recurrence~free survival.
Терапия болезни на поздних стадиях
Таргетная терапия
Unfortunately, despite a macroscopically complete resection, up to 50% of patients with primary GIST will recur at a median of 24 months [71]. An R0 or R1 resection is associated with 5year OS rates of 34–63% whereas R2 resection is associated with 5-year OS as low as 8%. Most recurrences occur along the peritoneal and serosal surfaces or within the liver. Lung and other soft tissue metastases only develop late in the course of progressive disease. True local recurrences are rare in the absence of a macroscopically incomplete resection, and most recurrences present as disseminated disease. The three risk factors most predictive of recurrence are tumor site of origin, primary tumor size, and mitotic count [72].
Imatinib mesylate, sunitinib malate, and regorafenib are approved for the treatment of metastatic GIST. Imatinib is the first-line therapy for advanced (unresectable primary or metastatic) GIST [73,74]. The starting dose is generally 400 mg once daily. In patients who develop progressive disease on 400 mg, dose escalation up to 400 mg twice daily may be effective; however, greater toxicity and more dose reductions are generally required at doses above 400 mg per day. Toxicities may include headache, fatigue, nausea, diarrhea, edema, muscle cramps, dermatitis, anemia, and neutropenia. Fortunately, >70% of side effects are mild to moderate in severity and often resolve with continuing therapy. Following imatinib treatment, partial responses or stable disease were observed in nearly 85% of patients. Median time to PFS and OS was 18–20 months and 51–55 months, respectively.
KIT mutation location correlates with response to imatinib therapy. Patients whose GIST harbored an exon 11 mutation had a much higher response rate of 72%, while only 32% of patients with exon 9 KIT mutants and 12% of wild-type KIT patients responded to imatinib [75]. Furthermore, the median event-free survival for patients whose tumors had mutations of exon 11 and were treated with imatinib was 22.5 months, compared to 6.6 months for patients with mutations in exon 9 [76]. In a meta-analysis of the two large phase III studies, a slight advantage in PFS was noted in patients initially treated with higher dose imatinib, but that advantage was essentially limited to patients with KIT exon 9 mutations, and there was no overall survival advantage [77].
Sunitinib is a multitargeted TKI whose targets include KIT, PDGFR, the ret proto-oncogene receptor (RET), Fms-like tyrosine kinase-3 receptor (Flt3), and vascular endothelial growth factor receptor (VEGFR1, VEGFR2, VEGFR3). If patients continue to progress on escalating doses of imatinib or do not tolerate imatinib, then second-line sunitinib is started. Initially dosed as 50 mg daily in a 4-week-on-2-week-off cycle, many oncologists now favor a continuous dosing regimen of 37.5 mg daily. A placebo-controlled phase III trial [70] demonstrated significant improvement in time to progression in patients treated with sunitinib compared to those treated with placebo (27.3 weeks vs 6.4 weeks, respectively), as well as PFS and OS.
Regorafenib recently demonstrated benefit as a third-line agent providing significant improvement in progression-free survival compared with placebo in a randomized, placebocontrolled, phase 3 trial [78].
Other TKIs evaluated or currently under investigation include pazopanib, dasatinib, masitinib, nilotinib, sorafenib, and vatalanib among others. Crenolanib is a novel PDGFRA kinase inhibitor undergoing evaluation in GIST patients whose tumors carry a PDGFRA D842V mutation resistant to most other kinases available [79]. Pazopanib is a multitargeted receptor TKI and was recently shown in a randomized, open-label phase II study to improve progression-free survival of patients with advanced GIST resistant to imatinib and sunitinib [80]. Other potential non-TKI molecules for targeted therapy include heat shock protein-90 [81], mammalian target of rapamycin (mTOR) [82], histone deacetylase [83], and insulin-like growth factor type I receptor [84].
The French randomized imatinib discontinuation study BFR14 demonstrated that patients with GIST on imatinib who stop imatinib therapy after 1 year, 3 and 5 years had a much higher rate of disease progression than those who continued on therapy [85–87]. Therefore, once metastatic disease develops, patients should remain on some therapy indefinitely.
Хирургия
Исследователи преследуют стратегию агрессивной циторедуктивной хирургии у пациентов с распространенными метастатическими GIST при TKI терапии, учитывая тот факт, что патологические полные ответы редки (<5% пациентов), большинство пациентов имели длительные периоды частичного ответа или стабильного заболевания на иматинибе, а ответ на иматиниб не поддерживается бесконечно (среднее время до прогрессирования, 18-24 месяца). Когда развивается лекарственная устойчивость, прогрессирование заболевания может проявляться либо в ограниченной (прогрессирование в одной опухолевой области, другие опухолевые депозиты показывают стойкий ответ на TKI), либо в генерализованной (прогрессирование более чем в одной области) манере.
Many single-institution retrospective studies have documented the PFS and OS rates following extensive cytoreductive surgery in patients with advanced GIST treated with TKI therapy. The goal for advanced or metastatic GISTs is to perform a macroscopically complete (R0 or R1) resection when safely possible. However, the disease frequently may be too extensive to be completely resected, in which case progressing lesions are preferentially removed. The best results are generally observed in patients whose disease is still responsive to TKI therapy at the time of surgery (78% in patients with responsive disease compared to 7% of patients with generalized progression, P <0.0001) [88]. Furthermore, the ability to remove all macroscopic disease is greatest in patients demonstrating ongoing response to TKI therapy (bulky residual disease remained postoperatively in only 4% of patients with responsive disease compared to 43% of patients with generalized progression). This seems more applicable to patients undergoing surgery while still on imatinib, and less relevant for patients undergoing surgery on sunitinib, where selection bias may cloud the impact of response to therapy on eventual outcomes [89].
Even though cytoreductive surgery is feasible, there is still no evidence that outcomes are superior or even equal to those for patients who continue on TKI therapy without surgery. Conversely, patients with generalized progression have not been shown to derive any benefit from cytoreductive surgery and are best treated nonoperatively. Nevertheless, these patients may need urgent surgery for palliative or emergency purposes if obstruction or hemorrhage occurs.
Наблюдение
Patients who have had resection of a primary GIST should undergo a history, physical examination, and abdomen/pelvis CT scan with intravenous contrast every 3–6 months during the first 3–5 years, and then annually thereafter according to the NCCN consensus panel recommendations [90,91]. CT scans of the head, chest, or other extra-abdominal areas are unnecessary given the rarity of pulmonary or extra-abdominal metastases. Screening using routine PET scans is also unnecessary. Imaging intervals of 3–6 months are standard for patients in the first 5 years of post-treatment follow-up, with less frequent annual evaluation thereafter given that most recurrences occur within the first 5 years after surgery. At present there are no specific serum-based markers in routine use to detect recurrent GIST, though this is an active area of development [92,93].
Литература
1 Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol 2002;33(5):484–95.
2 Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33(5):459–65.
3 Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;23;279(5350):577–80.
4 FDA.gov (Internet) Food and Drug Administration 2008 (updated December 7, 2010, cited December 23, 2008). Available from: http://www.fda.gov/Safety/MedWatch/ SafetyInformation/ucm2553333.htm
5 Perez EA, Livingstone AS, Franceschi D, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg 2006;202(4):623–9.
6 Bamboat AM, DeMatteo RP. Updates on the management of gastrointestinal stromal tumors. Surg Oncol Clin N Am 2012; 21:301–16.
7 Rubin JL, Sanon M, Taylor DC, et al. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med 2011;4:121–30.
8 Sшreide K, Sandvik OM, Sшreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39–46.
9 Benesch M, Wardelmann E, Ferrari A, Brennan B, Verschuur A. Gastrointestinal stromal tumors (GIST) in children and adolescents: a comprehensive review of the current literature. Pediatr Blood Cancer 2009;53(7):117–9.
10 Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol Oncol Clin North Am 2009;23(1):15–34.
11 Antonescu CR. Gastrointestinal stromal tumor (GIST) pathogenesis, familial GIST, and animal models. Semin Diagn Pathol 2006;23(2):63–9.
12 Li FP, Fletcher JA, Heinrich MC, et al. Familial gastrointestinal stromal tumor syndrome: phenotypic and molecular features in kindred. J Clin Oncol 2005;23(12):2735–43.
13 Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiyomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med 1977;296(26):1517–8.
14 Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med 2009;266(1):43–52.
15 Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 2011;24(1):147–51.
16 Andersson J, Sihto H, Meis-Kindblom JM, et al. NF1associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol 2005;29(9):1170–6.
17 Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol 2005;18:475–84.
18 Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetics study of 45 cases. Am J Surg Pathol 2006;30:90–6.
19 Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res 2008;14:4550–5.
20 Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. J Surg Oncol 2008;97(4):350–9.
21 Chaudhry UI, DeMatteo RP. Management of resectable gastrointestinal stromal tumor. Hematol Oncol Clin Noth Am 2009;23(1):79–96.
22 Patnaik S, Jyotsnarani Y, Rammurti S. Radiological features of metastatic gastrointestinal stromal tumors. J Clin Imaging Sci 2012;2:43.
23 Bley TA, Tittelbach-Helmrich D, Baumann T, et al. Sliding multislice MRI for abdominal staging of rectal gastrointestinal stromal tumours. In Vivo 2007;21(5):891–4.
24 Voiosu T, Voiosu A, Rimbas M, Voiosu R. Endoscopy: possibilities and limitations in the management of GIST of the upper GI tract. Rom J Intern Med 2012;50(1):7–11.
25 Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56(6):1757–62.
26 Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7(6):507–19.
27 Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol 2007;38(5):679–87.
28 Tarn C, Merkel E, Canutescu AA, Shen W, Skorobogatko Y, Heslin MJ, et al. Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: therapeutic implications through protein modeling. Clin Cancer Res 2005;11(10):3668–77.
29 Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn Pathol 2006;23(2):91–102.
30 Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008;53(3):245–66.
31 Andre C, Hampe A, Lachaume P, et al. Sequence analysis of two genomic regions containing the KIT and the FMS receptor tyrosine kinase genes. Genomics 1997;39(2): 216–26.
32 Liu FY, Qi JP, Xu FL, Wu AP. Clinicopathological and immunohistochemical analysis of gastrointestinal stromal tumor. World J Gastroenterol 2006;12(26):4161–5.
33 Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23(23):5357–64.
34 Panteleo MA, Astolfi A, Nannini M, et al. Differential expression of neural markers in KIT and PDGFRA wild-type gastrointestinal stromal tumours. Histopathology 2011;59(6):1071–80.
35 Gill AJ. Succinate dehydrogenase (SDH) and mitochondrial driven neoplasia. Pathology 2012;44(4):285–92.
36 Patil DT, Rubin BP. Genetics of gastrointestinal stromal tumors: a heterogeneous family of tumors? Surg Pathol Clin 2015;8(3):515–24.
37 Hostein I, Faur N, Primois C, Boury F, Denard J, Emile JF, et al. BRAF mutation status in gastrointestinal stromal tumors. Am J Clin Pathol 2010;133(1):141–8.
38 Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23(2):70–83.
39 Gold JS, Gцnen M, Gutiйrrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009;10(11):1045–52.
40 Andersson J, Bьmming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology 2006;130(6):1573–81.
41 DeMatteo RP, Maki RG, Agulnik M, et al. Gastrointestinal stromal tumor. In: MB Amin (ed) AJCC Cancer Staging Manual, Eighth Edition. New York: Springer, 2017.
42 Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant /adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0123/ACRIN 6665. J Surg Oncol 2009;99(1):42–7.
43 DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double blind, placebo-controlled trial. Lancet 2009;373 (9669).
44 Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307(12):1265–72.
45 Casali PG, Cesne AL, Velasco AP, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33(36):4276–83.
46 Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/recurrent operable gastrointestinal stromal tumors: long-term follow – up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19(4):1074–80.
47 McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16(4):910–9.
48 Hohenberger P, Langer C, Wendtner CM, et al. Neoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label phase II study in KITor PDGFRApositive tumors. J Clin Oncol 2012;30(suppl; abstr 10031).
49 Raut CP, Ashley SW. How I do it: surgical management of gastrointestinal stromal tumors. J Gastrointest Surg 2008;12(9):1592–9.
50 Fairweather M, Raut CP. Surgical management of GIST and intra-abdominal visceral leiomyosarcomas. J Surg Oncol 2015;111(5):562–9.
51 Fujimoto Y, Nakanishi Y, Yochimura K, Shimoda T. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 2003;6(1):39–48.
52 Prakash S, Sarran L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol 2005;27(4):179–87.
53 Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. Pediatric-type gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol 2011;35(4):495–504.
54 DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localized, primary gastrointestinal stromal tumour: a randomized, double-blind, placebo-controlled trial. Lancet 2009;373(9669):1097–104.
55 Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Human Pathol 2006;37(12):1527–35.
56 Cai R, Ren G, Wang DB. Synchronous adenocarcinoma and gastrointestinal stromal tumors in the stomach. World J Gastroenterol 2013;19(20):3117–23.
57 Yan Y, Li Z, Liu Y, et al. Coexistence of gastrointestinal stromal tumors and gastric adenocarcinomas. Tumour Biol 2013;34(2):919–27.
58 Agaimy A, Wьnsch PH, Hofstaedter F, et al. Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 2007;31(10):113–20.
59 Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243(6):738–45; discussion 745–7.
60 Otani Y, Furukawa T, Yoshida M, et al. Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 2006;139(4):484–92.
61 Shu ZB, Sun LB, Li JP, Li YC, Ding DY. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Chin J Cancer Res 2013;25(2):175–82.
62 De Vogelaere K, Hoorens A, Haentijens P, Delvaux G. Laparoscopic versus open resection of gastrointestinal stromal tumors or the stomach. Surg Endosc 2013;27(5): 1546–54.
63 Lee PC, Lai PS, Yang CY, et al. A gasless laparoscopic technique of wide excision for gastric gastrointestinal stromal tumor vs open method. World J Surg Oncol 2013;11:44.
64 Dematteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) Intergroup Phase 2 Trial. Ann Surg 2013;258(3):422–9.
65 Reichardt P, Joensuu H, Blay JY. New fronts in the adjuvant treatment of GIST. Cancer Chemother Pharmacol 2013;72(4):715–23
66 Zhan WH, Wang PZ, Shao YF, et al. Efficacy and safety of adjuvant post-surgical therapy with imatinib in gastrointestinal stromal tumor patients with high risk of recurrence: interim analysis from a multicenter prospective clinical trial. (Article in Chinese.) Zhonghua Wei Chang Wai Ke Za Zhi 2006;9(5):383–7.
67 Kang YK, Kang BW, Im SA, et al. Two-year adjuvant imatinib mesylate after complete resection of localized, high-risk GIST with KIT exon 11 mutation. Cancer Chemother Pharmacol 2013;71(1):43–51.
68 DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localized, primary gastrointestinal stromal tumour: arandomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104.
69 Raut CP, Espat J, Maki RG, et al. PERSIST-5: five year extended treatment with adjuvant imatinib for patients with intermediate/high risk primary gastrointestinal stromal tumor (GIST). 2017 ASCO Annual Meeting. J Clin Oncol 2017;35:(suppl; abstr 11009).
70 Demetri, GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet 2006;368(9544): 1329–38.
71 DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231(1):51–8.
72 DeMatteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112(3):608–15.
73 Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26(4):626–32.
74 Verweij, J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364(9440):1127–34.
75 Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21(23):4342–9.
76 Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42(8):1093–103.
77 Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta analysis of 1,640 patients. J Clin Oncol 2010;28(7):1247–53.
78 Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomized, placebo-controlled, phase 3 trial. Lancet 2013;381(9863):295–302.
79 Heinrich MC, Griffith D, McKinely A, et al. Crenolanib inhibits the drug resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res 2012;18(16):4375–84.
80 Mir O, Cropet C, Toulmonde M, Le Cesne A, Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A, Italiano A. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. The Lancet Oncology. 2016 May 31;17(5):632–41.
81 Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res 2006;66(18):9153–61.
82 Schцffski P, Reichardt P, Blay JY, Dumez H, Morgan JA, Ray-Coguard I, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol 2010;21(10):1990–8.
83 Floris G, Debiec-Rychter M, Sciot R, Stefan C, Fieuws S, Machiels K, et al. High efficacy of panobinostat towards human gastrointestinal stromal tumors in a xenograft mouse model. Clin Cancer Res 2009 Jun 15;15(12):4066–76.
84 Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U.S.A. 2008;105(24):8387–92.
85 Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25(9):1107–13.
86 Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomized phase 3 trial. Lancet Oncol 2010;11:942–9.
87 Blay JY, Adenis A, Ray-Coquard I, Cassier PA, Le Cesne A. Is there a role for discontinuing imatinib in patients with advanced gastrointestinal stromal tumour? Current Opn Oncol 2009;21(4):360–6.
88 Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24(15): p. 2325–31.
89 Raut CP, Wang Q, Manola J, Morgan JA, George S, Wagner AJ, et al., Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib maleate. Ann Surg Oncol 2010 Feb;17(2): 407–15.
90 Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010 Apr;8 Suppl 2:S1–41.
91 Von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, et al. Clinical practice guidelines in oncology. Soft tissue sarcoma V 1.2017. December 21, 2016. Nccn.org. accessed January 12, 2017.
92 Demetri, GD, Jeffers M, Reichardt P, Yoon-Koo K, Jean-Yves B, Rutkowski P, et al. Mutational analysis of plasma DNA from patients (pts) in the phase III GRID study of regorafinib (REG) versus placebo (PL) in tyrosine kinase inhibitor (TKI)refractory GIST: Correlating genotype with clinical outcomes. J Clin Oncol 31, 2013 (suppl:abstr 10503).
93 Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousovб M, Holubel L. Tumor markers in colorectal cacer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers (EGTM) 2014 guidelines update. Int J Cancer. 2014;134(11):2513–22.