The American Cancer Society’s Oncology in Practice: clinical management (2018)
Edited by American Cancer Society
Заболеваемость и смертность
Рак легкого является наиболее часто диагностируемым раком во всем мире с 1,8 миллионами новых случаев каждый год. Это составляет около 13% всех случаев рака в мире. Приблизительно с 1,6 миллионами смертей в год рак легких также является основной причиной смертности от рака во всем мире [1, 2]. Среди мужчин наиболее распространенным злокачественным новообразованием является рак легких, у женщин заболеваемость раком легких превышает только рак молочной железы и колоректальный рак. Оценочные показатели заболеваемости раком легких в более развитых странах составляют 18,6 на 100 000 женщин в год и 47,4 на 100 000 мужчин в год. Соответствующие показатели для менее развитых стран составляют 11,1 и 27,8 для женщин и мужчин соответственно. За последние два десятилетия смертность, связанная с раком легких у мужчин, снизилась в западных странах, но быстро растет в развивающихся странах. Тем не менее, у женщин заболеваемость и смертность от рака легкого продолжает расти в большинстве регионов мира. В США, по оценкам, в 2017 году будет диагностировано 222 500 случаев рака легких, и приблизительно 155 870 смертей будут вызваны раком легких [3]. В Европе ежегодно диагностируется 417 000 инцидентов рака легких с приблизительно 367 000 случаев смерти ежегодно [4]. За последние 30 лет в Китае выросла смертность от рака легких на 465% [5]. Приблизительно с 500 000 новых случаев ежегодно рак легких является наиболее распространенным раком в Китае как у мужчин, так и у женщин. Вследствие роста распространенности курения сигарет в развивающихся странах, по оценкам, к 2030 году большинство инцидентов рака легких будет наблюдаться за пределами США и Европы.
Факторы риска
Курение сигарет является наиболее распространенным фактором риска развития рака легких. Почти 85% пациентов с раком легких в прошлом курили табачные изделия. Среди них примерно 50% являются бывшими курильщиками, которые определены как некурящие в течение по меньшей мере 12 месяцев до вынесения диагноза рака легких. Риск развития рака легких пропорционален количеству выкуриваемых за день сигарет, а совокупная продолжительность курения у пациентов с историей курения более 20–30 пачек лет считается высоким риском возникновения рака легких. Хотя распространенность курения сигарет в США снижается, он растет угрожающими темпами в развивающихся странах и странах третьего мира. Следовательно, число случаев рака легких, диагностируемых ежегодно, вероятно, возрастет в течение следующих нескольких десятилетий. Отказ от курения связан с постепенным снижением риска развития рака легких, хотя он не достигает уровня никогда не курящего человека. Поскольку менее чем у 20% заядлых курильщиков развивается рак легких, генетическая восприимчивость к раку легких также, по-видимому, является риском. Женщины, видимо, подвержены более высокому риску развития рака легких по сравнению с мужчинами. В последние годы растет число никогда не курящих с диагнозом рак легких. Опухоли у этих людей с большей вероятностью могут иметь определенные генетические изменения, такие как мутации в гене рецептора эпидермального фактора роста (EGFR) и перестройка в гене киназы анапластической лимфомы (ALK) [6]. Вторичная экспозиция табачному дыму (сэконд-хэнд курение) является еще одним фактором риска, который способствует почти 1% всех случаев рака легких.
Профессиональная экспозиция асбесту является известным фактором риска развития рака легких [7, 8]. Подсчитано, что у пациентов без истории курения риск развития рака легких при воздействии асбеста в четыре раза выше. Курение сигарет оказывает аддитивное воздействие на повышение риска рака легких, ассоциированного с экспозицией асбесту [9]. Хотя использование асбеста запрещено почти в 50 странах мира, оно растет в Китае, Индии, России и многих других странах. Агентство по охране окружающей среды (Environmental Protection Agency, EPA) и Всемирная организация здравоохранения считают все виды асбеста канцерогенными. Между воздействием асбеста и развитием рака легких существует задержка в несколько десятилетий. Риск развития рака легких связан с длительностью воздействия асбеста, количеством и типом асбестового волокна.
Экспозиция радону также вовлечена в развитие рака легких [10]. Радон образуется в результате радиоактивного распада урана. В соответствии с оценкой EPA, в некоторых географических регионах воздействие радона в домашних хозяйствах является высоким и способствует почти 20 000 новых случаев рака легких в год [11]. EPA рекомендует снижение уровня радона до <4 пикокюри/л воздуха для минимизации риска развития рака легких. Простые корректирующие методы доступны для редукции экспозиции радону выше этого порога. Воздействие ионизирующего излучения в виде лечебного облучения или частых диагностических рентгенографических тестов также ассоциировано с более высоким риском развития рака легких. Промышленное воздействие металлов, таких как мышьяк, никель, хром и общее загрязнение воздуха, — ассоциировано с более высоким риском развития рака легких. Нет известных семейных генетических синдромов, связанных с раком легких.
Патология
Исторически рак легкого широко подразделяется на немелкоклеточный рак легкого (NSCLC) и мелкоклеточный рак легкого (SCLC), на основании отличительного поведения и ответа на химиотерапию между этими двумя субсетами пациентов. NSCLC включает аденокарциному, плоскоклеточную карциному и крупноклеточную карциному. За последние несколько лет найдены большие отличия между различными субгистологиями NSCLC, и большой акцент сделан на идентификацию подтипов из диагностических биопсийных образцов.
Аденокарцинома — наиболее распространенный гистологический подтип рака легкого. Она постепенно увеличивалось в инциденте, опередив плоскоклеточный рак за последние два десятилетия. В США аденокарцинома представляет почти 50% всех случаев рака легкого. Аденокарцинома имеет больший потенциал к отдаленному метастазированию по сравнению с плоскоклеточной гистологией. Никогда не курившие, у которых развивается рак легких, чаще всего имеют подтип аденокарциномы. С 2020 года используется новая система классификации аденокарциномы легкого [12]. В рамках этой системы аденокарцинома делится на преинвазивный, минимально инвазивный и инвазивный типы (Таблица 1.1). Атипичная аденоматозная гиперплазия относится к локальному пролиферативному поражению, состоящему из атипичных пневмоцитов II типа или клеток Клара (Clara) и размером <5 мм. Аденокарцинома in situ (AIS) относится к поражениям размером менее 3 см, которые не имеют каких-либо инвазивных характеристик. Ранее она называлась бронхиолоальвеолярным раком. Поражения ≤3 см с преобладающим лепидическим паттерном (то есть ростом вдоль альвеолярных структур) и инвазией <5 мм в наибольшем измерении называют минимально инвазивной аденокарциномой (МИА). AIS и MIA имеют> 95% 5-летнюю выживаемость в случае хирургической резекции. Инвазивная аденокарцинома составляет почти 90% инцидентов аденокарциномы. Основываясь на преобладающей модели роста, она классифицируется как лепидическая, ацинарная, папиллярная, микропапиллярная или солидная с преобладанием продукции муцина. В дополнение к морфологическим особенностям, иммуногистохимические исследования помогают установить гистологический подтип NSCLC. Образцы аденокарциномы, как правило, положительны для цитокератина 7, напсина А и транскрипционного фактора щитовидной железы-1 (TTF-1) и отрицательны для цитокератина 20. [13]. TTF-1 считается сильным маркером аденокарциномы на основании позитивности почти в 75–85% случаев [14].
Таблица 1.1. IASLC/ATS/ERS классификация аденокарциномы легкого в резекционных образцах.
- Прединвазивные поражения
- Атипичная аденоматозная гиперплазия
- Аденокарцинома in situ (≤3 см ранее BAC)
- Немуцинозная
- Муцинозная
- Смешанная муцинозная/немуцинозная
- Минимально инвазивная аденокарцинома (≤3 см лепидическая преобладающая опухоль с ≤5 миллиметровой инвазией)
- Немуцинозная
- Муцинозная
- Смешанная муцинозная/немуцинозная
- Инвазивная аденокарцинома
- Лепидическая преобладающая (ранее немуцинозный BAC паттерн, с > 5-мм инвазией)
- Ацинарная преобладающая
- Папиллярная преобладающая
- Микропапиллярная преобладающая
- Солидная преобладающая с продукцией муцина
- Варианты инвазивной аденокарциномы
- Инвазивная муцинозная аденокарцинома (ранее муцинозная BAC)
- Коллоидная
- Фетальная (низко и высоко злокачественная)
- Энтерическая
ATS, American Thoracic Society; BAC, bronchioloalveolar carcinoma; ERS, European Respiratory Society; IASLC, International Association for the Study of Lung Cancer [28].
В США заболеваемость плоскоклеточным раком легких снижается, вероятно, вследствие изменения привычки курения популяции. Плоскоклеточные опухоли часто расположены в центре и почти всегда наблюдаются у пациентов с историей курения. Плоскоклеточная дисплазия и плоскоклеточный рак in situ являются прединвазивными поражениями, которые могут развиваться в инвазивный рак. Большинство плоскоклеточных опухолей положительно окрашиваются на p63 и p40 маркеры; эти маркеры могут тестироваться на диагностических образцах рака легких, у которых отсутствует видимая плоскоклеточная дифференциация на рутинно окрашенных стеклах. Группа маркеров, включающая TTF-1, p63 и p40, все чаще анализируется в диагностических образцах пациентов с раком легкого для выявления гистологического подтипа [14].
Крупноклеточная карцинома составляет 3–4% NSCLC и характеризуется высокой митотической скоростью, некрозом и морфологическими чертами NSCLC [15, 16]. Опухоли положительно окрашиваются на нейроэндокринные маркеры, такие как хромогранин А и синаптофизин. Точный диагноз этого гистологического подтипа требует избыточности биопсийной ткани. Крупноклеточная карцинома характеризуется агрессивным клиническим течением и низкой выживаемостью даже на ранней стадии заболевания. Крупноклеточная карцинома сильно ассоциирована с историей курения.
SCLC диагностируется примерно в 13% случаев рака легких в США. Заболеваемость SCLC постепенно снижалась в течение последних трех десятилетий в США. SCLC сильно ассоциирован с курением и редко встречается у некурящих. Патологический диагноз устанавливается с помощью световой микроскопии, которая демонстрирует характерные особенности, такие как высокая частота митозов и некрозов. Диагностическое обследование SCLC включает иммуноокрашивание TTF-1, хромогранина, синаптофизина и CD-56. Примерно 15% образцов SCLC имеют смешанную морфологию с компонентами NSCLC [15, 17].
Молекулярная патология
В последние годы был выявлен ряд молекулярных нарушений при раке легких (рис. 1.1) [18]. Многие из них представляют собой мишени для терапии, и поэтому получение адекватной опухолевой ткани для проведения молекулярных исследований является важным компонентом диагностической диагностики рака легких. Гетерогенность рака легких с точки зрения симптоматики и клинического течения была распознана в течение длительного времени. Сегодня, лучшее понимание молекулярных особенностей, которые объясняют неоднородность, ведет к индивидуализированному подходу к лечению. При аденокарциноме легкого почти две трети пациентов имеют онкогенную мутацию, которая потенциально может таргетироваться специфическими агентами. Наиболее распространенными среди них являются мутации KRAS, EGFR, B-RAF, HER-2, PIK3CA и перестройки генов ALK, RET и ROS1. K-RAS мутации присутствуют у ~25% пациентов с аденокарциномой легкого и часто ассоциированы с курением сигарет. Наиболее распространенные сайты мутаций в K-RAS включают кодоны 12, 13 и 61, что ведет к замене аминокислоты [19]. Это приводит к нарушению GTPase активности, что обеспечивает конститутивную активацию RAS сигналинга. Прогностическое значение K-RAS мутации у пациентов с раком легких, является спорным, несмотря на ранние сообщения, что это предвещает плохой общий исход и низкую сенситивность к химиотерапии.
Mutations in EGFR are observed in nearly 15% of White lung cancer patients and 40% of Asians. Deletion mutation in exon 19 and a point mutation in exon 21 are the most common EGFR mutations. These mutations are located in the tyrosine kinase-binding domain of the receptor and result in constitutive activation of the pathway, leading to proliferation, evasion of apoptosis and angiogenesis. Patients with EGFR activating mutations derive robust clinical benefits with EGFR tyrosine kinase inhibitors (TKI) [20, 21]. Nearly 60% of patients with an EGFR mutation will develop a secondary mutation in exon 20 (T790M) upon continued exposure to an EGFR TKI [22]. This mutation is the most common mechanism of resistance to EGFR TKI therapy, but can also be found de novo in certain patients with lung adenocarcinoma along with an exon 19 or 21 mutation prior to exposure to EGFR TKI therapy. In approximately 5% of patients with lung adenocarcinoma, gene rearrangement involving ALK is observed. This fusion gene results in activation of downstream signals that can be inhibited by specific ALK kinase inhibitors. Crizotinib, an ALK inhibitor, induces objective tumor response in nearly two-thirds of patients [23]. It is noteworthy that EGFR and K-RAS mutations and ALK gene rearrangement are mutually exclusive. ALK gene rearrangement is detected by the fluorescent in situ hybridization (FISH) test using the Vysis break-apart assay. Immunohistochemistry can be used as a screening step before conducting the FISH test. Other fusion abnormalities involving the RET and ROS1 genes are each present in 1% of lung adenocarcinoma specimens [24]. In addition to these molecular events, p53 mutation and LKB1 loss are commonly observed in lung adenocarcinoma patients [24].
Плоскоклеточный рак имеет совершенно другой спектр молекулярных аномалий. Недавние исследования в рамках Cancer Genome Atlas (TCGA) указывают на типичные мутации, включая TP53, потерю PTEN, PIK3CA, KEAP1, DDR2 и RB1 [25]. Амплификация гена рецептора фактора роста фибробластов (FGFR) также наблюдается в 10–20% случаев плоскоклеточного рака легких. Многие из этих нарушений обеспечивают потенциальные возможности для таргетной терапии. В SCLC типичные генетические изменения включают мутации RB1 и TP53, которые наблюдаются у ~90% и ~50% пациентов соответственно. Доступность очень сложных методов секвенирования генома позволяет обнаруживать до сих пор неопознанные молекулярные аномалии и, таким образом, находить новые терапевтические мишени в раке легких. Благодаря сегодняшней технологии все чаще можно проводить «мультиплексное» тестирование ряда молекулярных маркеров при ограниченном объеме образцов ткани. Руководства, выпущенные IASLC, рекомендуют регулярное тестирование на EGFR мутацию и ALK транслокацию для всех недавно диагностированных пациентов с аденокарциномой легкого. При сквамозноклеточной гистологии рутинное молекулярное тестирование пока не рекомендуется.
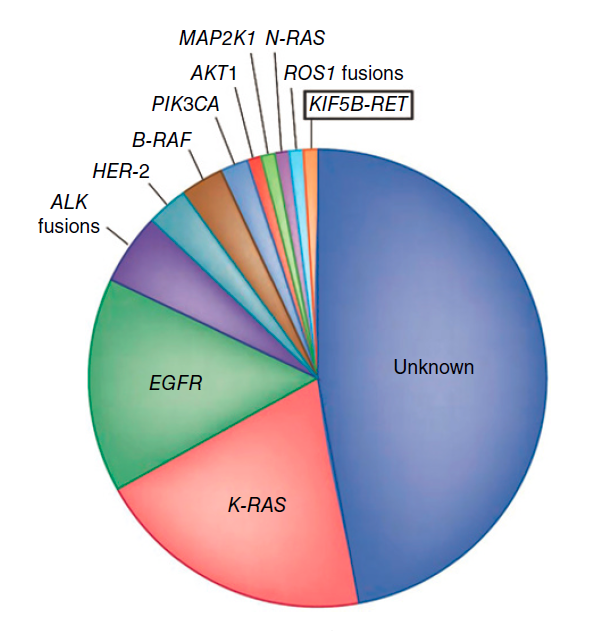
Рисунок 1.1. Молекулярные драйверы аденокарциномы легкого.
Диагноз
Presenting symptoms of lung cancer include cough, dyspnea, pain, hemoptysis, and weight loss. Since most patients with lung cancer have other tobacco-related cardiopulmonary diseases, these overlapping symptoms often result in a delay in diagnosis of the underlying malignancy. Symptoms could also result from local invasion or metastasis of the tumor such as headache, bone pain, bronchial obstruction, etc. Paraneoplastic syndromes associated with lung cancer include syndrome of inappropriate anti-diuretic hormone (SIADH), hypercalcemia, pulmonary hypertrophic osteoarthropathy, Eaton-Lambert myasthenic syndrome (ELMS), and Cushing syndrome. Some of the paraneoplastic syndromes are associated with specific histologies; hypercalcemia is common in squamous cell carcinoma, whereas SIADH, ELMS, and Cushing syndrome are common in SCLC. Diagnosis of lung cancer at an early stage is often made as an incidental finding during evaluation for other conditions. With the advent of computed tomography (CT) screening, it is anticipated that a greater subset of patients with lung cancer will be detected before the onset of symptoms.
In patients with clinical or radiographic findings suspicious of lung cancer, CT scans of the chest and abdomen are indicated to determine the location of the primary tumor, involvement of mediastinal lymph nodes, and spread to other anatomic sites. The most common sites of metastasis with lung cancer include mediastinal lymph nodes, contralateral lung, liver, adrenal gland, bones, and the brain. Imaging of the brain is recommended to evaluate for metastasis in patients with suggestive symptoms and signs, or those with lung adenocarcinoma >3 cm and evidence of mediastinal nodal involvement. Magnetic resonance imaging (MRI) or CT scan with contrast are acceptable modalities to evaluate for brain metastasis. Radionuclide study of the bones is indicated in patients with symptoms of bone pain or an unexplained elevation in serum alkaline phosphatase level. Positron emission tomography (PET) utilizing 18fluorodeoxyglucose (FDG) is included as part of staging for lung cancer in patients with localized lung cancer or for evaluation of solitary pulmonary nodules. The use of an FDG-PET scan to assess response to anticancer therapy and in surveillance following curative therapy is not recommended. An MRI scan of the chest may be useful in determining invasion of surrounding structures such as the brachial plexus in patients with tumors involving the superior sulcus of the lung.
A biopsy is necessary to establish diagnosis, and in recent years, to conduct molecular studies (for NSCLC) that can guide therapy. The most accessible site with the least invasive method is the preferred approach to obtaining diagnostic tissue. A fineneedle aspiration procedure is often adequate for establishing the diagnosis of lung cancer, and can be accomplished by a transthoracic approach or by bronchoscopy. However, the yield from a fine-needle aspiration is often inadequate to conduct molecular studies. Therefore, in recent years, a core-needle biopsy to obtain sufficient tissue is recommended for patients with suspected lung cancer. For patients presenting with pleural or pericardial effusions, transthoracic aspiration of the fluid is sufficient to establish the diagnosis and to complete staging workup. Cell blocks prepared by centrifuging the fluid, and processing the pellet as a histological specimen, can be used to conduct molecular studies, though the success rate depends on the number of viable cancer cells in the specimen. The diagnostic yield of pleural fluid in patients with a malignant effusion is approximately 50–70% [26]. In instances where repeated aspiration of pleural fluid is nondiagnostic, a video-assisted thoracoscopy procedure might be necessary to establish the diagnosis. For patients with localized lung tumors that are suspicious for cancer, it is reasonable to proceed with surgical resection without a diagnostic biopsy if all other potential etiologies are excluded.
In recent years, with the utilization of molecularly targeted therapies, understanding the mechanism of resistance has emerged as an important determinant of subsequent therapies. Therefore, obtaining additional tumor biopsies at various timepoints during the course of treatment is recommended.
Ранняя детекция
Десятилетия исследований по скринингу лиц с высоким риском для раннего выявления рака легких наконец-то увенчались успехом. National Lung Cancer Screening Trial рандомизировало субъектов для скрининга с низкодозными CT сканами или рентгенограммами грудной клетки, которые были выполнены в начале исследования и через 1 и 2 года после регистрации [27]. Положительные результаты наблюдалось у ~25% и 7% пациентов, прошедших скрининг с CT и рентгенограммой грудной клетки, соответственно. Среди пациентов с положительной компьютерной томографией 96,4% были признаны ложными после соответствующего дополнительного обследования. Адверзивные реакции наблюдались редко, у ~1,5% пациентов с аномальным сканированием развивались осложнения, связанные с дальнейшим диагностическим обследованием. Скрининг с ежегодными низкодозным CT обследованием индивидуумов c высоким риском был ассоциирован с 20%-ым уменьшением в летальности от рака легких. Смертность от всех причин (all-cause mortality) также снизилась на 6,7%. ~80% пациентов, у которых был диагностирован рак легких низкодозной CT, имели заболевание I, II или IIIA стадии, которое поддается лечебной терапии. Эти результаты в настоящее время привели к принятию низкодозной CT для раннего выявления рака легких основными соответствующими медицинскими организациями, включая American Cancer Society.
Стадийность
Стадия является наиболее важной детерминантой прогноза для пациентов с раком легких. Седьмая редакция American Joint Committee on Cancer (AJCC) и Union for International Cancer Control (UICC) система, введенная в 2010 году, использовалась до конца 2017 года [28]. 8-я редакция AJCC стадийной системы включает ряд изменений в 7-м издании и введена с 1 января 2018 года [29] (таблица 1.2). Дескрипторы основаны на анализе почти 95 000 случаев из 16 стран мира. Заметные изменения включают введение новых дескрипторов «T» и «M» в систему TNM. Индивидуальные T-дескрипторы определяются размером опухоли: <1 см (T1a), 1-2 см (T1b), 2–3 см (T1c), 3–4 см (T2a), 4–5 см (T2b) ), 5–7 см (Т3) и> 7 см (Т4). Нодальная стадийность также пересмотрена, и новые дескрипторы включают в себя: один узел N1 (N1a), несколько узлов N1 (N1b), один узел N2 без N1 (N2a1), один узел N2 с N1 (N2a2), несколько узлов N2 (N2b), и N3. Пациенты с метастатической болезнью классифицируются на основе количества и локализации метастаза в: злокачественный плевральный или перикардиальный выпот, отдельный опухолевый узел в контрлатеральной доле (M1a), одиночный внегрудной метастаз в одном органе (M1b) и множественные экстраторакальные метастазы (M1c ) (Рис. 1.2) [30]. Эта стадийная система применима как к NSCLC, так и к SCLC.
Таблица 1.2. TNM стадийная система рака легкого Американского объединенного комитета по раку (American Joint Committee on Cancer, AJCC).
| Определение первичной опухоли (T) | ||
| T категория | T критерий | |
| TX | Первичная опухоль не может быть оценена, или опухоль, доказанная присутствием злокачественных клеток в мокроте или бронхиальном лаваже, но не визуализируемая образом или бронхоскопией | |
| T0 | Нет свидетельства первичной опухоли | |
| Tis |
Карцинома in situ
Плоскоклеточная карцинома in situ (SCIS) Аденокарцинома in situ (AIS): аденокарцинома с чистым лепидическим паттерном, ≤3 см в наибольшем диаметре |
|
| T1 | Опухоль ≤3 см в наибольшем измерении, окруженная легкой или висцеральной плеврой, без бронхоскопических свидетельств инвазии более проксимально, чем долевой бронх (т.е. не в главном бронхе) | |
| T1mi | Минимально инвазивная аденокарцинома: аденокарцинома ≤3 см в наибольшем измерении с преимущественно лепидическим паттерном и инвазией ≤5 мм в наибольшем измерении | |
| T1a | Опухоль ≤1 см в наибольшем измерении. Поверхностная распространяющаяся опухоль любого размера, инвазивный компонент которой ограничен стенкой бронха и может проксимально простираться до главного бронха, также классифицируется как T1a, но эти опухоли встречаются редко | |
| T1b | Опухоль> 1 см, но ≤2 см в наибольшем измерении | |
| T1c | Опухоль> 2 см, но ≤3 см в наибольшем измерении | |
| T2 |
Опухоль> 3 см, но ≤5 см или имеющая любую из следующих черт:
• Вовлекает главный бронх независимо от расстояния до киля, но без вовлечения киля • Инвазирует в висцеральную плевру (PL1 или PL2) · Ассоциирована с ателектазом или обструктивным пневмонитом, которая распространяется на корень легкого, включая часть или все T2 опухоли легкого с этими признаками, классифицируются как T2a, если ≤4 см или если размер не может быть определен, и T2b, если> 4 см, но ≤5 см |
|
| T2a | Опухоль> 3 см, но ≤4 см в наибольшем диаметре | |
| T2b | Опухоль> 4 см, но ≤5 см в наибольшем диаметре | |
| T3 | Опухоль> 5 см, но ≤7 см в наибольшем измерении или прямо инвазирующее любое из следующего: париетальную плевру (PL3), грудную стенку (включая опухоли верхней борозды), диафрагмальный нерв, париетальный перикард; или отдельные опухолевые узлы в той же доле, что и первичная опухоль | |
| T4 | Опухоль> 7 см или опухоль любого размера, поражающая одно или несколько из следующего: диафрагму, средостение, сердце, магистральные сосуды, трахею, рецидивирующий нерв гортани, пищевод, тело позвонка или киль; отдельные опухолевые узлы в ипсилатеральной доле, отличной от первичной опухоли | |
| Определение региональных лимфатических узлов (N) | ||
| N категория | N критерий | |
| NX | Региональные лимфатические узлы не могут быть оценены | |
| N0 | Нет метастазов в региональные лимфоузлы | |
| N1 | Метастазы в ипсилатеральные перибронхиальные и/или ипсилатеральные лимфатические узлы и интрапульмонарные узлы, включая вовлеченных путем прямого распространения | |
| N2 | Метастазы в ипсилатеральный медиастинальный и/или субкаринальный лимфатический узел(ы) | |
| N3 | Метастазы в контралатеральный медиастинальный, контралатеральный хиларный, ипсилатеральный или контралатеральный разносторонний или надключичный лимфатический узел(ы) | |
| Определение отдаленного метастазирования (M) | ||
| M категория | M критерий | |
| M0 | Нет отдаленных метастазов | |
| M1 | Отдаленный метастаз | |
| M1a | Отдельные опухолевые узелки в контралатеральной доле; опухоль с плевральными или перикардиальными узлами или злокачественным плевральным или перикардиальным экссудатом. Большинство плевральных (перикардиальных) экссудатов с раком легких являются результатом активности опухоли. Однако у некоторых пациентов многократные микроскопические исследования плевральной (перикардиальной) жидкости отрицательны для опухоли, и жидкость не является кровью и не является экссудатом. Если эти элементы и клиническое суждение диктуют, что выпот не связан с опухолью, выпот следует исключить как дескриптор стадии | |
| M1b | Одиночный внегрудной метастаз в одном органе (включая вовлечение одного нерегионального узла) | |
| M1c | Множественные внегрудные метастазы в одном или нескольких органах | |
AJCC прогностические стадийные группы
| Стадийная группа | T | N | M |
| Оккультная карцинома | TX | N0 | M0 |
| 0 | Tis | N0 | M0 |
| IA1 | T1mi | N0 | M0 |
| IA1 | T1a | N0 | M0 |
| IIB | T1a | N1 | M0 |
| IIIA | T1a | N2 | M0 |
| IIIB | T1a | N3 | M0 |
| IA2 | T1b | N0 | M0 |
| IIB | T1b | N1 | M0 |
| IIIA | T1b | N2 | M0 |
| IIIB | T1b | N3 | M0 |
| IA3 | T1c | N0 | M0 |
| IIB | T1c | N1 | M0 |
| IIIA | T1c | N2 | M0 |
| IIIB | T1c | N3 | M0 |
| IB | T2a | N0 | M0 |
| IIB | T2a | N1 | M0 |
| IIIA | T2a | N2 | M0 |
| IIIB | T2a | N3 | M0 |
| IIA | T2b | N0 | M0 |
| IIB | T2b | N1 | M0 |
| IIIA | T2b | N2 | M0 |
| IIIB | T2b | N3 | M0 |
| IIB | T3 | N0 | M0 |
| IIIA | T3 | N1 | M0 |
| IIIB | T3 | N2 | M0 |
| IIIC | T3 | N3 | M0 |
| IIIA | T4 | N0 | M0 |
| IIIA | T4 | N1 | M0 |
| IIIB | T4 | N2 | M0 |
| IIIC | T4 | N3 | M0 |
| IVA | Любой T | Любой N | M1a |
| IVA | Любой T | Любой N | M1b |
| IVB | Любой T | Любой N | M1c |
Лечение
The outcomes for patients with lung cancer have improved significantly in recent years. This is a result of improvements in staging, better surgical and radiation therapy techniques, availability of newer and more effective systemic therapeutic agents, understanding of molecular characteristics and the ability to individualize therapy, and improved supportive care measures. Improvement in survival has been noted for every stage of lung cancer in the past two decades. A team approach for the management of lung cancer including thoracic surgeons, radiation oncologists, medical oncologists, interventional pulmonologists, pathologists, radiologists, and nursing support is critical to develop and deliver appropriate treatments. Surgery, radiation therapy, and systemic therapy are all used for lung cancer.
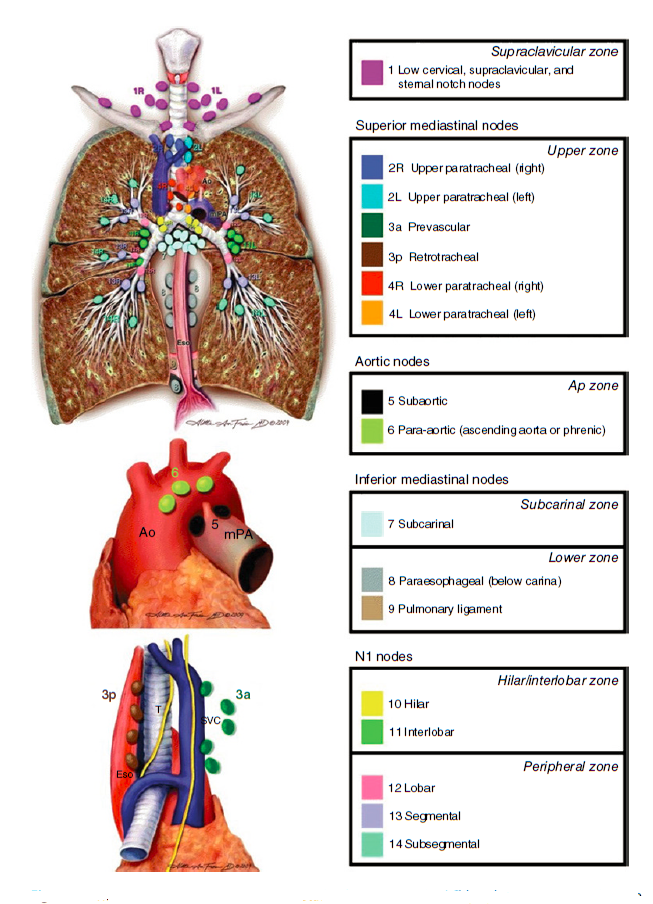
Фигура 1.2. Карта лимфатических узлов Международной ассоциации по изучению рака легких (International Association for the Study of Lung Cancer, IASLC), включая предлагаемую группировку лимфатических узлов в «зоны» с целью прогностического анализа.
NSCLC
Хирургия
Surgical management plays a major role in the treatment of early-stage lung cancer [31]. Patients with stages I, II, and selected stage III are considered potential candidates for surgical resection. Since most lung cancer patients suffer from smoking-related medical illness, nearly 40% of patients with early-stage lung cancer are not candidates for surgery due to limiting comorbid conditions. The commonly used parameters for inoperability include a baseline FEV1 of <40%, a predicted postoperative FEV1 of <30%, or a severely limited diffusion capacity. Such patients are referred to as ‘medically inoperable’ despite the presence of localized disease [32].
The first step in managing localized lung cancer is to stage the mediastinal lymph nodes. Cervical mediastinoscopy allows for staging of most relevant mediastinal lymph node stations with the exception of subaortic and para-aortic lymph nodes (levels 5 and 6). Cervical mediastinoscopy is associated with a mortality rate of <1%. Sampling of lymph nodes in levels 5 and 6 requires either a video-assisted thoracoscopy or anterior mediastinostomy. In recent years, endobronchial ultrasound-guided biopsy of mediastinal nodes has allowed for noninvasive staging, and to sample nodes in patients who have already undergone mediastinoscopy. With the advent of PET-CT scans, mediastinoscopy and endobronchial ultrasound are selectively utilized in the preoperative assessment based on the likelihood of nodal involvement. For peripheral tumors that are not associated with mediastinal adenopathy and do not have FDG uptake in the nodes, many surgeons advocate proceeding with surgical resection and sampling mediastinal nodes intraoperatively. However, for patients with nodes that are positive on the PET scan, sampling is strongly recommended before surgery. The false positivity rate for a PET scan in the mediastinum for patients with localized lung cancer is approximately 20%. The likelihood of nodal involvement in patients with a negative PET scan is approximately 5–15%.
Lobectomy is the standard surgical procedure of choice for patients with localized lung cancer. If anatomical resection cannot be achieved with lobectomy, either a bilobectomy or pneumonectomy might be necessary. Sleeve resection refers to removing the tumor along with the bronchus and anastomosing the remaining ends of the bronchial tree [33]. Surgical resection can be achieved by either performing a thoracotomy or by the video-assisted thoracic surgery approach. The latter is gaining wider use due to lower morbidity and a faster recovery from surgery. It also allows for better tolerance of postoperative systemic therapy. The ability to achieve an R0 resection is critical to surgical management of early-stage NSCLC. If this is not deemed feasible during preoperative workup, then surgery should not be attempted. For patients with positive surgical margins, a re-resection should be attempted whenever feasible. If not, postoperative radiotherapy should be administered.
Sublobar resections are not recommended due to the higher risk of local recurrence. An exception to this rule is for patients with peripheral tumors <2 cm in size, where studies have demonstrated excellent outcomes. An ongoing study is comparing sublobar resection to standard lobectomy and will likely provide definitive answers to this critical issue. Two important aspects of surgical management of lung cancers have been addressed in recent clinical trials. A randomized comparison of mediastinal lymph node dissection to nodal sampling demonstrated comparable outcomes for patients with NSCLC [34]. Another study compared sublobar resection followed by placement of I125 brachytherapy to the tumor bed to surgery alone in patients who are not candidates for standard lobectomy [35]. There was no difference in overall survival between the two groups and therefore, the brachytherapy approach is not recommended. Tumors involving the superior sulcus are managed with preoperative chemoradiotherapy. The decision to perform surgery for these tumors depends on extent of local invasion, involvement of the brachial plexus and mediastinal lymph node involvement.
The role of surgery in the management of stage III NSCLC with mediastinal nodal involvement continues to be controversial. Surgery alone is associated with poor outcome for stage III disease. In a randomized study, patients with N2-positive disease who underwent chemoradiotherapy followed by surgery did not have improved survival compared to chemoradiotherapy alone [36]. There was an especially high rate of postoperative mortality for patients who underwent pneumonectomy following chemoradiotherapy. Therefore, trimodality therapy is not recommended for patients who require a pneumonectomy. For patients with multistation N2 disease or bulky nodal disease, surgical resection is not recommended. It appears that clearance of the mediastinal node after induction therapy might be the most important predictor of benefit from surgical resection. This calls for restaging the mediastinum after induction therapy if surgery is contemplated.
The role of surgery in patients with oligometastatic disease can be considered under certain situations. Surgical resection of both the primary and a solitary brain metastasis has resulted in 5-year survival rates of approximately 20% [37]. However, this approach cannot be recommended for patients with mediastinal nodal involvement. Similar approaches to resect lung primary and solitary metastasis at other distant sites are not recommended for routine care.
Лучевая терапия
Лучевая терапия является важной частью мультимодальной терапии NSCLC. Она используется как в режиме куративной терапии для стадии III болезни, так и в паллиации на IV стадии болезни. В последние годы лучевая терапия успешно прошла испытания у пациентов с медицински нерезектабельным заболеванием I стадии. За последние два десятилетия были достигнуты значительные улучшения в проведении лучевой терапии. Это позволяет использовать меньший размер радиационного поля, тем самым уменьшая облучение нормальной ткани и повышая эффективность лечения опухоли. Техника respiratory gating позволяет проводить лучевую терапию опухоли независимо от фазы дыхания. Стереотаксическая радиотерапия организма (stereotactic body radiotherapy, SBRT) включает доставку высокодозной радиации к ограниченному объему опухоли после стереотаксического направления.
- I и II стадия NSCLC
SBRT has emerged as an effective treatment option for patients with T1 and T2 node negative tumors that are not candidates for surgery due to medical comorbid illness. Delivery of SBRT over three to five fractions is associated with a nearly 90% local control rate [38]. SBRT is appropriate for peripheral tumors, whereas for centrally located tumors, studies are presently ongoing to determine the appropriate dose and the safety of this approach. The highly promising results with SBRT for localized NSCLC have prompted studies to compare SBRT to surgical resection even in medically fit patients. Studies are also underway to combine SBRT with systemic therapy for early-stage NSCLC.
Radiation therapy is indicated for patients with positive surgical margins following surgery for early-stage NSCLC. A dose of 60 Gy is administered for patients with microscopic margins, whereas for those with residual macroscopic disease doses of up to 66 Gy are administered in once daily fractions of 1.8–2 Gy each. For patients with negative surgical margins, there is no role for adjuvant radiotherapy. A meta-analysis published in 1998 reported a detrimental effect for patients treated with postoperative radiotherapy, especially for those with N0 and N1 disease [39]. Patients with N2 disease demonstrated a favorable survival trend with radiotherapy. This has also been observed in an analysis of the national Surveillance, Epidemiology and End Results database in the US [40]. Based on this, a prospective study is presently underway in Europe to compare postoperative radiation to observation in patients with surgically resected N2 disease. In this setting, radiotherapy is delivered to the bronchial stump, ipsilateral hilum, and involved mediastinal lymph node stations to a dose of approximately 50.4–54 Gy.
III стадия NSCLC
Radiation therapy is an essential component of multimodality therapy for management of stage III NSCLC. Surgery is appropriate for patients with T3N1 disease, but for patients with involvement of the mediastinal lymph nodes, administration of radiotherapy with chemotherapy results in improved outcomes. A subset of N2 positive patients might benefit from multimodality therapy that includes surgery. In such settings, radiation can be administered with chemotherapy as neoadjuvant therapy followed by surgical resection. Radiation therapy dose consists of 45 Gy of once daily fractions when given in the preoperative setting. More recently, a radiation dose of 60 Gy has been piloted with acceptable safety results. Potential candidates for the tri-modality therapy approach include stage IIIA patients who have single station or microscopic lymph node involvement and disease that is amenable to resection with lobectomy or bilobectomy.
For patients with stage III disease who are not appropriate for surgical resection, thoracic radiotherapy with concomitant chemotherapy is the recommended treatment. This category includes patients with bulky mediastinal disease, involvement of contralateral or supraclavicular nodes (N3) and direct invasion of major structures such as the vertebrae, trachea, major blood vessels, or esophagus by the primary tumor (T4). Radiotherapy is administered to a dose of 60–66 Gy in once daily fractions as part of definitive therapy for stage III disease. Five-year survival rates of approximately 20–25% have been reported with combined chemoradiotherapy in this setting [41]. The main adverse events associated with this approach include esophagitis and pneumonitis. The risk of pneumonitis depends on the extent of normal lung tissue and the dose of radiation received by the normal lung tissue in the radiation port. Radiation-related pneumonitis can occur in the acute setting immediately following the radiotherapy course or after 6–9 months.
Several efforts to improve upon standard chemoradiotherapy havebeenundertakeninthepasttwodecades. Hyperfractionated radiotherapy with administration of two to three fractions/day has demonstrated favorable results over once-daily fractionation, particularly in squamous cell carcinoma [42, 43]. However, the logistical constraints associated with multiple daily fractions have limited the adoption of this approach. Another strategy studied in stage III disease involved utilization of higher doses of radiation of up to 74 Gy in once-daily fractions. A randomized study conducted by the RTOG comparing 74 Gy to 60 Gy demonstrated inferior survival with the higher dose [44]. Therefore, 60–66 Gy remains the standard radiation dose for stage III NSCLC.
- IV стадия NSCLC
Лучевая терапия используется для паллиации определенных симптомов у пациентов с распространенным NSCLC. Основными показаниями являются лечение метастазов в головной мозг, уменьшение обструкции бронхов, кровохарканье и контроль боли. При метастазах в головной мозг облучение всего мозга равно 30–37,5 Гр, разделенных на 10–15 фракций. Стереотаксическая радиохирургия (SRS) используется вместо облучения всего мозга для пациентов с метастазами небольшого объема в количестве одного-трех поражений. SRS может также использоваться для поражений головного мозга, которые развиваются после радиотерапии всего мозга. SRS значительно улучшает выживаемость пациентов с метастазами в головной мозг. Контроль боли в области костных метастазов или поражения грудной стенки может достигаться коротким курсом лучевой терапии. Доза и количество фракций определяется местом и размером поражения. Компрессия спинного мозга является чрезвычайной ситуацией, которая часто разрешается внешней лучевой терапией. Хирургическая декомпрессия используется в определенных обстоятельствах, когда неврологическое осложнение является ранним, и пациент имеет хорошо контролируемое системное заболевание, с последующей лучевой терапией.
Системная терапия
Systemic therapy refers to the use of cytotoxic therapy, immunotherapy, or molecularly targeted agents. Systemic therapy was initially developed for patients with advancedstage lung cancer. This has subsequently been extended to the treatment of earlier stages of lung cancer. The high propensity for metastasis of lung cancer cells provides the rationale for the use of systemic therapy even for patients with earlier stages of the disease who are treated with local therapies. A number of effective and well-tolerated cytotoxic agents have been developed over the past three decades that are utilized for routine care of patients with lung cancer (Table 1.3).
- Распространенный/метастатический NSCLC
In patients with advanced-stage NSCLC, systemic chemotherapy improves both survival and quality of life. Platinum-based combination regimens were superior to supportive care alone in randomized trials and were associated with modest improvement in overall survival [45, 46]. Cisplatin was the first platinum compound developed in NSCLC. Subsequently carboplatin was studied as a better-tolerated alternative to cisplatin. The use of cisplatin is associated with adverse events such as nausea, emesis, nephrotoxicity, and neurotoxicity. The availability of highly effective antiemetic agents has greatly improved the tolerability of cisplatin. Carboplatin is associated with ease of administration in the outpatient setting. The dose-limiting toxicity of carboplatin is thrombocytopenia. Both compounds are efficacious in advanced NSCLC. In several randomized trials, the use of combination regimens was associated with a higher response rate and improved survival over cisplatin alone. Etoposide, vinblastine, vindesine, vinorelbine, taxanes, gemcitabine, irinotecan, and pemetrexed, have all been combined with cisplatin or carboplatin in the treatment of advanced NSCLC. The two-drug combination regimens have also been compared to monotherapy with a nonplatinum compound. For instance, a phase 3 study compared the combination of carboplatin and paclitaxel to paclitaxel alone for first-line therapy of advanced NSCLC [47]. The efficacy outcomes were all more favorable with the combination, though toxicity was also increased. This led to the adoption of combination chemotherapy as the recommended approach for the treatment of advanced NSCLC.
Таблица 1.3. Часто используемые для лечения рака легкого химиотерапевтические агенты.
| Немелкоклеточный рак легкого | Мелкоклеточный рак легкого |
| Цисплатин | Цисплатин |
| Карбоплатин | Карбоплатин |
| Паклитаксел | Этопозид |
| Nab-паклитаксел | Иринотекан |
| Доцетаксел | Топотекан |
| Гемцитабин | |
| Пеметрексед (несквамозная гистология) | |
| Иринотекан | |
| Винорелбин |
A meta-analysis of randomized trials to compare the efficacy of cisplatin to carboplatin in advanced-stage NSCLC demonstrated comparable survival [48]. When cisplatin was used in combination with a third-generation cytotoxic agent such as taxanes, gemcitabine, or vinorelbine, there was a statistically significant albeit modest improvement in survival. Cisplatinbased regimens were associated with a numerically higher incidence of treatment-related deaths. Taken together, though cisplatin-based regimens have a slight advantage in efficacy over carboplatin-based regimens in advanced NSCLC, the latter is associated with a favorable tolerability profile. With palliation being the goal of therapy in advanced NSCLC, carboplatinbased regimens have found wider adoption due to their favorable therapeutic index.
Several partner agents for platinum have demonstrated efficacy in advanced NSCLC. Paclitaxel, docetaxel, gemcitabine, irinotecan, pemetrexed, and vinorelbine have all demonstrated single-agent activity in advanced NSCLC with single-agent response rates of approximately 10–20%. Each of these agents can be given in combination with platinum with acceptable tolerability profile. In randomized trials, the efficacy across platinum-based combination regimens was similar. The ECOG 1594 trial randomized 1,206 advanced NSCLC patients to treatment with cisplatin–paclitaxel, cisplatin–docetaxel, cisplatin–gemcitabine or carboplatin–paclitaxel [49]. The median survival was comparable for all four regimens, and the differences were primarily in toxicity. The median survival was approximately 8 months and the median progression-free survival was 3.5–4.2 months for all four regimens. The 1-year survival rate was approximately 40%. The main toxicities associated with the paclitaxel–carboplatin regimen were neuropathy and myelosuppression. Thrombocytopenia was common with the cisplatin–gemcitabine regimen, while the cisplatin–docetaxel regimen was associated with myelosuppression. Based on E1594 and other contemporary studies, the choice of any one of these chemotherapy agents for front-line treatment is made upon consideration of toxicity, patient preference, schedule, and cost. Combinations of three cytotoxic agents are not recommended due to a higher toxicity burden and lack of incremental benefit.
- Роль гистологии в выборе химиотерапии
Until recently, chemotherapy regimens were considered to be suitable for all histological subtypes of NSCLC. This notion was dispelled in a phase 3 study of cisplatin–pemetrexed versus cisplatin–gemcitabine that was compared in patients with advanced-stage NSCLC [50]. The pemetrexed-based regimen was noninferior to the comparator for the overall patient population, but was associated with a superior survival for patients with nonsquamous histology. In patients with squamous histology, the gemcitabine-based regimen was superior. Consequently, the use of pemetrexed should be restricted to patients with nonsquamous histology only. The relative efficacy of taxanes versus pemetrexed in nonsquamous histology has not been compared directly. In a recent randomized study, patients were given either carboplatin and pemetrexed or carboplatin and paclitaxel. Patients on both treatment groups received bevacizumab, a monoclonal antibody against the vascular endothelial growth factor (VEGF) in addition to chemotherapy. There was no difference in overall survival between the two treatment groups [51]. Based on these observations, taxane-based and pemetrexed-based regimens are both appropriate for the treatment of patients with nonsquamous histology.
The US Food and Drug Administration (FDA) recently approved nanoparticle albumin-bound paclitaxel (nab-paclitaxel) for the treatment of advanced NSCLC. In contrast to the standard formulation, use of nab-paclitaxel does not require premedication and is not associated with hypersensitivity reactions. The incidence of neuropathy is also lower with the use of nab-paclitaxel. In a direct comparison to carboplatin and paclitaxel, the carboplatin-nab-paclitaxel regimen was associated with a favorable response rate, when given to patients with advanced NSCLC, though survival was not improved [52]. The improvement in response rate appeared to be restricted to squamous histology. The variable efficacy of pemetrexed and nab-paclitaxel based on histology should be considered when chemotherapy is selected for first-line treatment of advanced NSCLC.
- Поддерживающая терапия
The duration of chemotherapy for advanced-stage NSCLC has been debated and studied closely. Four to six cycles of combination therapy are considered optimal in the first-line setting. Continuation of combination treatment beyond this duration is associated with cumulative toxicities, but no tangible therapeutic benefit. More recently, a strategy of single-agent maintenance therapy has been successfully developed. In one approach referred to as ‘switch maintenance’, patients who derive clinical benefit with a platinum-based combination for four cycles are treated with an alternative cytotoxic or targeted agent that has not been previous administered. The ‘continuation maintenance’ strategy involves continuing the nonplatinum agent beyond the four cycles for patients who experience either an objective response or stable disease with combination therapy. Pemetrexed is the only cytotoxic agent that has demonstrated survival advantage as maintenance therapy in advanced NSCLC [53]. It has been tested both as continuation and switch maintenance therapies in advanced nonsquamous histology. The improvement in survival was of similar magnitude in randomized trials. Based on these observations, pemetrexed has been approved for maintenance therapy in the US and Europe. Erlotinib, an EGFR inhibitor, also extends survival when used as maintenance therapy in patients who received a platinum-based combination for four cycles [54]. The benefit was notable only for patients who experienced stable disease with combination chemotherapy. EGFR genotypic status was a significant determinant of efficacy of erlotinib, with a robust magnitude of progression-free survival benefit for patients with an activation mutation.
Pemetrexed and erlotinib are also efficacious when used as salvage therapy for patients with advanced NSCLC that experience disease progression during or after platinum-based chemotherapy. Therefore, the relative merits of using these agents as maintenance therapy versus after disease progression has been controversial. The benefits of maintenance therapy are counterbalanced by the toxicity, logistical, and cost factors. A ‘wait and watch’ approach after first-line therapy appears reasonable, though approximately 40% of the patients might never receive salvage therapy due to rapid disease progression or decline in performance status. For these reasons, careful discussion with the patients regarding the merits of maintenance therapy versus close observation is recommended.
- Спасительная терапия
Nearly all patients with advanced NSCLC will experience disease progression regardless of the extent of benefit with first-line chemotherapy. Salvage therapy for such patients provides modest improvement in survival. Docetaxel, given at a dose of 75 mg/m2 every 3 weeks, was the first proven agent in this setting. In randomized studies, docetaxel monotherapy was associated with improvements in survival when compared to best supportive care, and improved 1-year survival rate over first-generation cytotoxic agents [55]. Disease stabilization is observed in approximately 40% of patients, but objective response occurs in <10% with docetaxel in the salvage therapy setting. Pemetrexed is an alternative cytotoxic agent with proven efficacy as salvage therapy, but its use is restricted to patients with nonsquamous histology. In a randomized study, the overall survival associated with pemetrexed was noninferior to that with docetaxel [56]. However, the toxicity profile was better with pemetrexed as evidenced by lower incidence of fever with neutropenia and hospitalizations. EGFR inhibition with erlotinib, which was originally approved for salvage therapy of advanced NSCLC, is now only recommended for patients with EGFR sensitizing mutations [57].
The salvage therapy setting for advanced NSCLC has been substantially changed in the past year following the approval of three immune-check point inhibitors nivolumab, pembrolizumab, and atezolizumab. Nivolumab and pembrolizumab target the programmed death (PD-1) receptor, whereas atezolizumab targets PDL-1, a ligand for PD-1. Each one of these agents demonstrated superiority over docetaxel in improving survival when used as second-line therapy [58–61]. They were also associated with a favorable toxicity profile relative to chemotherapy. The salient efficacy data for these three agents are summarized in Table 1.4.
Таргетная терапия (Таблица 1.5)
- Антиангиогенная терапия
Approaches to inhibit angiogenesis as a therapeutic strategy have been extensively pursued in patients with advanced NSCLC. VEGF is a critical determinant of neoangiogenesis in the physiologic milieu and in cancer. Bevacizumab is a monoclonal antibody that binds and inhibits all active isoforms of VEGF. Building on strong preclinical observations, bevacizumab was studied in combination with standard chemotherapy for first-line therapy of advanced NSCLC [62]. The initial results were promising, though squamous histology was associated with a higher incidence of life-threatening hemoptysis. Further development of this agent was subsequently limited to nonsquamous histology. The ECOG 4599 study compared bevacizumab in combination with carboplatin and paclitaxel versus chemotherapy alone [63]. There was a significant improvement in overall survival (12.3 months vs 10.3 months) and progression-free survival (6.3 months vs 4.8 months) with the addition of bevacizumab. The notable adverse events included bleeding, hypertension, and proteinuria along with a higher risk of neutropenia. Another randomized study conducted in Europe failed to document a survival improvement with the addition of bevacizumab to cisplatin and gemcitabine, despite a modest improvement in progression-free survival [64]. In older individuals (age >70 years) bevacizumab appears to have a narrow therapeutic index due to higher risk of myelosuppression and bleeding [65]. All pivotal randomized trials performed with bevacizumab utilized it as maintenance therapy following six cycles of combination therapy. Therefore, the use of maintenance therapy with bevacizumab has been adopted in clinical practice for patients who receive it as part of the initial treatment regimen. The therapeutic value of maintenance bevacizumab has not been directly studied to date.
Таблица 1.4. Ингибиторы иммунных чекпоинтов как спасительная терапия.
| Агент | Частота ответа (%) | Median progression-free survival (months) | Median survival (months) |
|
Nivolumab
vs |
20 | 3.5 | 9.2 |
| Docetaxel (squamous histology) | 9 | 2.8 (HR 0.62, P <0.001) | 6.0 (HR 0.59, P <0.001) |
|
Nivolumab
vs |
19 | 2.3 | 12. 2 |
| Docetaxel (nonsquamous histology) | 12 | 4.2 (HR 0.92, P = 0.39) | 9.4 (HR 0.73, P = 0.002) |
|
Pembrolizumab (2 mg/kg)1
vs |
18 | 3.9 | 12.7 |
| Docetaxel | 18 | 4.0 (HR 0.88, P = 0.07) | 10.4 (HR 0.71, P = 0.0008) |
|
Atezolizumab
vs |
14 | 2.8 | 13.8 |
| Docetaxel | 13 | 4.0 (HR 0.95, P = 0.49) | 9.6 (HR 0.73, P = 0.0003) |
1 Study enrolled patients with PDL-1 expression >1%.
Таблица 1.5. Молекулярно таргетные агенты с доказанной эффективностью для рака легкого.
Рецептор эпидермального фактора роста
Обратимые ингибиторы: ерлотиниб, гефитиниб
Необратимые ингибиторы: афатиниб, осимертиниб
Моноклональное антитело: цетуксимаб, нецитумумаб
Киназа анапластической лимфомы
Кризотиниб, церитиниб, алектиниб
Антиангиогенная терапия
Бевацизумаб, рамуцирумаб
Ramucirumab, a monoclonal antibody against the VEGF receptor (R2), has proven efficacious as second-line therapy in combination with docetaxel [66]. A phase 3 study demonstrated modest gains in survival (10.5 months vs 9.5 months, HR 0.86) and progression-free survival (4.5 months vs 3.0 months, HR 0.76) for the combination compared to docetaxel alone. A small subset of patients in this study had received prior bevacizumab and appeared to derive benefit from a ramucirumab-based combination. The combination of docetaxel with ramucirumab has received approval from the US FDA for salvage therapy of advanced NSCLC. Other strategies to inhibit angiogenesis including small molecule inhibitors of VEGF tyrosine kinase and vascular disrupting agents have not been successful to date in advanced NSCLC. Efforts to identify biomarkers to predict benefit with bevacizumab and other antiangiogenic agents have been unsuccessful and have unquestionably restricted optimal utilization of these agents.
- EGFR ингибирование
Inhibition of EGFR is the first successful molecular treatment strategy in lung cancer. This has in no small measure contributed to the expanding role of targeted approaches and molecular classification of lung cancer. Initially, agents that target EGFR were evaluated based on preclinical observations of higher expression of the target protein in aggressive tumors. Objective response rates of 10–20% were noted with gefitinib and erlotinib, small molecule TKIs of EGFR. Subsequent studies demonstrated that patients with robust responses harbored an activation mutation in exons 19 or 21 of the EGFR [20, 21, 67, 68]. The mutations result in constitutive activation of the receptor and therefore the tumors are exquisitely sensitive to EGFR inhibition. EGFR activating mutations are exclusive to adenocarcinoma histology and occur at a higher frequency in women, never-smokers and patients with Asian ethnicity. In randomized studies of patients with an activating mutation, EGFR inhibition with either gefitinib or erlotinib was associated with an improvement in progression-free survival over platinumbased chemotherapy [69–71]. This has not translated into survival benefit, most likely due to most patients treated with chemotherapy subsequently receiving an EGFR inhibitor upon disease progression. Quality of life is also more favorable with EGFR inhibitors over chemotherapy in this setting. The importance of molecular testing before initiation of EGFR inhibitor therapy in first-line treatment is highlighted by the inferior outcomes in wild-type patients treated with targeted therapy. Afatinib, an irreversible EGFR TKI, has also demonstrated superiority over chemotherapy in patients with an activating mutation [72]. This agent is associated with a higher incidence of diarrhea relative to gefitinib and erlotinib. Another irreversible inhibitor, dacomitinib, is being compared to gefitinib in an ongoing phase 3 clinical trial.
The median progression-free survival with EGFR TKI in this setting is approximately 8–12 months. Mechanisms leading to resistance are increasingly being understood. A secondary mutation in exon 20 (T790) is responsible for resistance to EGFR TKI in nearly 60% of patients [22, 73]. Activation of alternate pathways such as MET signaling also contributes to resistance to EGFR inhibition.
Osimertinib, a third generation EGFR TKI, inhibits exon 19, 21, and T790M signaling. In early-phase clinical trials, osimertinib demonstrated a high response rate (65%) and median progression-free survival of 9–13 months for patients who developed acquired resistance through the T790M mechanism [74]. This agent has recently received accelerated approval from the US FDA and has emerged as the preferred agent for this patient subset. Osimertinib is under evaluation for front-line treatment of patients with EGFR mutations. The use of EGFR inhibitors in patients with earlier stages of the disease is not known, even for those with an activating mutation. Ongoing studies are evaluating the role of EGFR inhibition in patients with surgically resected NSCLC and those with locally advanced disease.
The use of combination chemotherapy with EGFR TKI cannot be recommended based on present experience. Cetuximab, a monoclonal antibody against EGFR, was associated with a modest improvement in overall survival when given in combination with cisplatin and vinorelbine for first-line treatment of advanced NSCLC [75]. Necitumumab, another monoclonal antibody against the EGFR, was recently approved for the treatment of patients with advanced-stage squamous cell lung cancer. A randomized study that compared the combination of cisplatin and gemcitabine given with or without necitumumab demonstrated modest improvements in survival and progression-free survival for the addition of the EGFR antibody [76]. The median overall survival with and without necitumumab were 11.5 months and 9.9 months respectively (HR 0.84, P = 0.01).
- ALK ингибиторы
Онкогенный потенциал перестройки гена, включающей киназу анапластической лимфомы в раке легкого, был описан в 2007 [77]. Слившийся ген следует из инверсии или транслокации частей EML4 (echinoderm microtubule-associated protein-like 4) гена с ALK геном. Другие партнеры по слиянию помимо EML4 были также описаны для ALK. Перестройка ALK гена наблюдается приблизительно у 5-7% пациентов с аденокарциномой легкого [78]. Clinical features associated with the ALK gene rearrangement include never-smokers, adenocarcinoma histology, signet ring features on histopathological evaluation, and younger age. Limited available data indicate that patients with ALK translocation respond poorly to conventional treatment options and might also be at higher risk of recurrent disease after surgical resection for early-stage NSCLC [79]. Crizotinib, an inhibitor of MET, ALK, and ROS1 tyrosine kinases has demonstrated a response rate of nearly 60% and a clinical benefit rate of 90% in ALK-positive NSCLC [23, 80, 81]. The median progression-free survival was 10 months in a phase 2 study for patients with ALK-positive advanced-stage NSCLC [82]. Based on these exciting data, the US FDA and the European Medicines Agency have both approved crizotinib for the treatment of patients with advanced-stage ALK-positive NSCLC. Crizotinib was compared to platinum-based chemotherapy in a phase 3 study which demonstrated higher response rate and median progression-free survival with crizotinib [83]. When compared to chemotherapy in the salvage therapy setting, critozinib was associated with a significant improvement in progression-free survival (7.7 months vs 3 months) and response rate (66% vs 20%) [81]. Interestingly, pemetrexed was associated with a favorable outcome compared to docetaxel in this patient population. Mechanisms of resistance to crizotinib include activation of either ALK-dependent or independent alternate pathways. A variety of secondary mutations have been described in patients who develop disease progression while on therapy with crizotinib. Ceritinib, a potent ALK inhibitor, has demonstrated a response rate of 60% in patients who developed disease progression during crizotinib therapy [84]. Alectinib, another second generation ALK inhibitor, is also effective for patients who progressed on crizotinib [85]. Both of these agents are also effective against brain metastasis. Other novel ALK inhibitors are also under development for management of crizotinib resistance or as primary therapy. The use of ALK inhibitors in the management of earlier stages of NSCLC is under investigation.
- Другие таргетные субпопуляции
The availability of advanced genomic technology has made it possible to identify new molecular ‘drivers’ in lung cancer. In lung adenocarcinoma, a fusion gene involving ROS1, observed in 1% of patients, also confers sensitivity to treatment with crizotinib [86, 87] Another fusion involving the RET gene has been identified in approximately 0.5–1% of patients [88–91]. Patients with mutations in BRAF appear to respond to therapy with dabrafenib, a BRAF inhibitor or the combination of dabrafenib and trametinib [92, 93]. These observations provide hope that the mutation status of patients can aid personalized treatment of patients with lung cancer. The Cancer Genome Atlas Project recently published results of gene sequencing studies in a cohort of patients with squamous cell lung carcinoma [25]. A number of potentially targetable mutations and other genetic abnormalities have been identified. Routine testing of patient tumor specimens for molecular targets is increasingly seen as a strategy to optimize treatment options for lung cancer.
Ингибирование иммунных чекпоинтов
Recent progress in targeting the immune pathways that regulate cancer has resulted in major therapeutic gains for a number of malignancies, including lung cancer. Activation of the PD-1 pathway results in T-cell exhaustion, thereby blunting the ability of the host immune system to eliminate the cancer cell. Agents that target the PD-1 pathway have now demonstrated anticancer effects in lung cancer, both as salvage therapy and first-line therapy for a subset of patients. Nivolumab and pembrolizumab, monoclonal antibodies that target PD-1, demonstrated superiority over docetaxel for salvage therapy of advanced NSCLC (Table 1.4) [58, 59, 61]. Both agents improved overall survival and were associated with lower incidence of grades 3/4 toxicity relative to docetaxel. Atezolizumab, a monoclonal antibody against PDL-1, also demonstrated similar benefits against docetaxel. These agents have supplanted docetaxel and have become the preferred second-line therapy for advanced NSCLC.
A recent study in the front-line setting for advanced NSCLC demonstrated superior survival and progression-free survival with pembrolizumab over platinum-based chemotherapy for a subset of patients with advanced NSCLC [94]. Patients with tumor PDL-1 expression >50% were chosen for this study, which represents approximately 25–30% of advanced NSCLC. The median progression-free survival was 10.3 months with pembrolizumab compared to 6 months with chemotherapy (HR 0.50, P <0.001). The overall survival hazard ratio was 0.60 favoring pembrolizumab. This has now led to the FDA approval of pembrolizumab for first-line therapy of advanced NSCLC for patients with tumors that have PDL1 expression >50%. This new paradigm shift in first-line therapy of NSCLC provides hope that the use of immune checkpoint inhibitors can be extended to other settings such as earlier stages of the disease to improve cure rates. Biomarkers to select patients for therapy are being studied. In addition, combination strategies to improve the efficacy of immune checkpoint inhibitors are also under development.
Менеджмент особых групп пациентов
Elderly patients represent a growing subset of lung cancer patients. In the US, the median age at diagnosis of lung cancer is 70 years [95]. Aging is associated with decline in physiological and vital organ function that impact tolerance of systemic therapy. In addition, it is particularly more important to consider the implications of therapy on physical function and quality of life of older patients. A number of elderly-specific studies have been conducted in NSCLC patients. Initially, single-agent chemotherapy was compared to supportive care and demonstrated improved survival [96]. In subsequent studies, for elderly patients with a good performance status, platinum-based combinations were superior to single-agent therapy [47, 97]. The use of three-drug combinations of cytotoxic agents is not recommended for older patients. However, the appropriate use of targeted agents in older patients might be associated with clinical benefit.
A high percentage of NSCLC patients present with significant symptoms that are associated with a poor performance status. The median survival for advanced NSCLC patients with a performance status of 2 (ECOG scale) is dismal at less than 4 months. Poor performance status limits the ability of patients to tolerate combination chemotherapy. Studies conducted exclusively in patients with a poor performance status indicate a favorable role for chemotherapy. In at least one randomized study, platinum-based combination therapy was superior to single-agent therapy [98]. It is important to consider the underlying cause of poor performance status in making treatment plans for this patient population. For those with limiting comorbid conditions, a less aggressive approach with single-agent chemotherapy might be more appropriate. For those with targetable mutations, appropriate targeted therapy can be given regardless of the performance status given the greater potential for benefit.
Системная терапия NSCLC ранних стадий
Despite optimal surgery, recurrence of disease continues to be common for early-stage NSCLC. This is attributed to the presence of micrometastasis in early-stage NSCLC. The use of systemic therapy following surgery was recently proven to be associated with an improvement in 5-year survival rate [99]. In randomized trials, cisplatin-based two-drug combination regimens were compared to observation following surgery for early-stage NSCLC [100–102]. For patients with stage II and IIIA NSCLC, there was an absolute improvement of survival of 5–15% at 5 years. This corresponds to a relative risk reduction of approximately 30% with adjuvant chemotherapy. The consistent survival benefit observed across multiple trials has resulted in the adoption of four cycles of cisplatin-based adjuvant therapy as the standard of care for early-stage NSCLC. In stage IA disease, however, potential benefits of chemotherapy are outweighed by the risks, and there is an overall detrimental effect. For patients with stage IB disease, post-hoc analysis from two randomized trials revealed that survival improvement with adjuvant therapy was restricted to patients with tumor size >4 cm [102, 103]. This observation is yet to be validated in prospective trials. The cisplatin–vinorelbine combination has been the regimen commonly utilized in clinical trials of adjuvant therapy. The availability of better tolerated newer agents that are effective in the treatment of advanced NSCLC such as taxanes, gemcitabine, and pemetrexed, have prompted physicians to use these agents with cisplatin in early-stage NSCLC. Presently there are no effective tools to predict the risk of recurrent disease beyond pathological stage. It is hoped that the use of adjuvant chemotherapy could be tailored to patients at high risk of recurrence, based on genomic or proteomic markers.
Локально распространенный NSCLC
Chemotherapy has a proven role in combination with radiotherapy in the management of stage III disease that is not amenable to surgical resection. Initially, chemotherapy was used sequentially with radiotherapy and resulted in an improved overall survival over radiotherapy alone. Both local and systemic control was improved with the combined modality approach. Subsequent studies demonstrated a modest superiority for concomitant administration of chemotherapy over sequential therapy [41, 104]. Both cisplatin and carboplatin-based regimens have been utilized for combined modality therapy and are associated with modest survival results. The relative merits of cisplatin versus carboplatin in this setting have not been studied. The regimen of cisplatin and etoposide allows for administration of full systemic dose of chemotherapy with radiotherapy. The widely used regimen of carboplatin and paclitaxel involves administration of lower ‘radiosensitizing’ doses of the two agents with radiotherapy followed by consolidation therapy with two cycles at regular doses. The latter approach has a favorable tolerability profile compared to cisplatin-based regimens. Esophagitis and pneumonitis are the most notable toxicities with the combined modality treatment of locally advanced NSCLC. The use of induction or consolidation chemotherapy in other settings has not resulted in improved survival. With modern combined chemoradiotherapy, cure rates of nearly 20–25% are achieved in locally advanced NSCLC.
SCLC
SCLC is characterized by initial sensitivity to systemic chemotherapy, though recurrence of disease is common regardless of the extent of initial response. Approximately two-thirds of the patients present with extensive-stage SCLC, defined as the presence of metastatic disease outside the chest or large volume thoracic disease that cannot be treated with radiotherapy. The overall goal of treatment of extensive-stage disease is palliation. The median survival of untreated extensive-stage SCLC is less than 2 months. The use of platinum-based chemotherapy results in a response rate of approximately 50–70% and a median survival of 9–11 months. Improvement in symptoms and functional status are commonly observed within a few days of initiation of systemic chemotherapy in SCLC. The regimen of cisplatin and etoposide is considered the standard approach for the treatment of SCLC. Carboplatin is considered an acceptable alternative in the treatment of extensive-stage disease. Four cycles of chemotherapy are considered optimal, though it can be extended for up to six cycles in responding patients. There is no proven role for maintenance therapy after combination chemotherapy. Despite the extent of initial response, disease recurrence develops in a median of 4–5 months. Disease that progresses either during or within 90 days of administration of cisplatin-based chemotherapy is referred to as “refractory” relapse. Disease recurrence outside this window of time represents a “sensitive” subgroup of patients who might benefit from subsequent salvage treatment options. The use of other approaches such as high-dose chemotherapy, alternating chemotherapy regimens, dose-dense therapy and three-drug combination regimens are not associated with improvement in survival [105]. In the Japanese patient population, the regimen of cisplatin and irinotecan has demonstrated superior results over cisplatin and etoposide. However, cisplatin–irinotecan was not superior to standard therapy in Western patients.
Salvage therapy has yielded modest results in relapsed SCLC, but the benefit is restricted to “sensitive” relapse. Topotecan is the only agent to demonstrate clinical benefit in relapsed SCLC. In a randomized study, topotecan was associated with favorable symptomatic parameters, but overall survival was not improved [106]. The response rate for topotecan in this setting is approximately 20%. Several novel agents are presently being studied in efforts to improve the outcomes for SCLC. Molecularly targeted agents against known targets appear rational and provide hope for improved outcomes.
Radiotherapy is utilized in patients with limited-stage SCLC. Cure can be achieved for approximately 30% of patients with limited-stage SCLC with combined modality therapy. Earlier initiation of radiotherapy appears to be superior to the delayed approach and has been adopted as the standard approach in fit patients. A randomized study demonstrated superior survival when 45 Gy of thoracic radiotherapy was given at twice daily fractions (BID) compared to the same dose given at one fraction per day along with cisplatin and etoposide chemotherapy [107]. An ongoing study will evaluate whether the 45 Gy of BID radiation is superior to 70 Gy of radiotherapy given once daily with concomitant chemotherapy for limited-stage SCLC.
Prophylactic cranial irradiation (PCI) is associated with a modest improvement in 5-year survival rate for patients with limited-stage SCLC that achieve a complete remission following combined modality therapy [108, 109]. This is due to the high risk of brain recurrence that is noted in patients with SCLC. Recent studies have demonstrated benefit with PCI even in patients with extensive-stage disease [110]. For patients who achieve a favorable response to combination chemotherapy, the use of PCI results in modest improvement in overall survival and reduced risk of recurrence in the brain. Based on this, PCI can be considered for appropriate patients with extensivestage SCLC.
The role of surgery is limited to those with peripheral lung lesions without mediastinal nodal involvement. It is estimated that fewer than 5% of patients with SCLC are candidates for surgical resection. In 10–15% of patients with SCLC, a mixed histology with NSCLC features are observed. These patients might present with local progression following combined modality therapy resulting from the NSCLC component. These patients may be considered for surgical resection in selected situations.
Treatment advances in SCLC have lagged behind those for NSCLC in the past two decades. Consequently, the survival outcomes for SCLC have not changed considerably during this time. A concerted effort to develop appropriate preclinical models to test new agents, genomic subcategorization of SCLC, and discovery of new systemic anticancer agents are necessary to improve outcomes for this aggressive disease.
Наблюдение и выживаемость
Survivorship has emerged as an important area of research as outcomes for lung cancer have improved in recent years. Increasing numbers of survivors following surgery or chemoradiotherapy provide the impetus to investigate important topics such as optimal surveillance, follow-up for second primary disease, managing long-term consequences of chemoradiotherapy, etc. The importance of smoking cessation cannot be overemphasized given the high risk of second primary tumors in lung cancer survivors. Patients should be provided with appropriate opportunities to receive counseling, smoking cessation, and behavioral therapy.
There is presently no standard approach for optimal radiographic and clinical follow-up in patients who undergo surgical resection or chemoradiotherapy. CT scans are commonly used for follow-up of these patients. However, the relative merits of CT scan versus chest radiograph, frequency of evaluation, and the role of FDG-PET scans are all important questions that should be answered in prospective clinical trials. For patients with advanced-stage disease, CT scans are used to assess response to therapy and are often performed every two to three cycles of treatment. Given the proven role for salvage therapy, patients who are in follow-up after combination chemotherapy should be closely followed for development of new symptoms or clinical deterioration in addition to periodic radiographic studies.
Respiratory therapy should be offered to patients with dyspnea following surgery or chemoradiotherapy. Since a high proportion of these patients also have smoking-related pulmonary diseases, referral to a pulmonologist should be considered in symptomatic patients. Overall, a team approach that includes supportive care personnel, oncologists, and appropriate additional specialists, should be utilized to ensure the return of lung cancer survivors to normalcy to the fullest extent possible.
Литература
1 Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108.
2 Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50.
3 Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin, in press
4 International Agency for Research on Cancer GLOBOCAN 2008 cancer fact sheet: lung cancer incidence and mortality worldwide. Available from URL: http://globocan.iarc.fr (accessed December 2013).
5 Wang YC, Wei LJ, Liu JT, Li SX, Wang QS. Comparison of cancer incidence between china and the USA. Cancer Biol Med 2012;9:128–32.
6 Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121–34.
7 Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 2013;34:205–16.
8 Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med 1955;12:81–6.
9 Balmes JR. Asbestos and lung cancer: what we know. Am J Respir Crit Care Med 2013;188:8–9.
10 Lantz PM, Mendez D, Philbert MA. Radon, smoking, and lung cancer: the need to refocus radon control policy. Am J Public Health 2013;103:443–7.
11 Pawel DJ, Puskin JS. The U.S. Environmental Protection Agency’s assessment of risks from indoor radon. Health Phys 2004;87:68–74.
12 Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244–85.
13 Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer 2002;86:1884–7.
14 Sterlacci W, Savic S, Schmid T, et al. Tissue-sparing application of the newly proposed IASLC/ATS/ERS classification of adenocarcinoma of the lung shows practical diagnostic and prognostic impact. Am J Clin Pathol 2012;137:946–56.
15 Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol 2010;21(Suppl 7):vii65–71.
16 Paci M, Cavazza A, Annessi V, et al. Large cell neuroendocrine carcinoma of the lung: a 10-year clinicopathologic retrospective study. Ann Thorac Surg 2004;77:1163–7.
17 Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184–97.
18 Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nat Med 2012;18:349–51.
19 Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201–5.
20 Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39.
21 Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500.
22 Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92.
23 Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693–703.
24 Cardarella S, Johnson BE. The impact of genomic changes on treatment of lung cancer. Am J Respir Crit Care Med 2013;188:770–5.
25 Aregbe AO, Sherer EA, Egorin MJ, et al. Population pharmacokinetic analysis of 17-dimethylaminoethylamino-17demethoxygeldanamycin (17-DMAG) in adult patients with solid tumors. Cancer Chemother Pharmacol 2012;70:201–5.
26 Kaifi JT, Toth JW, Gusani NJ, et al. Multidisciplinary management of malignant pleural effusion. J Surg Oncol 2012;105:731–8.
27 National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409.
28 Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–14.
29 Amin M, Edge S, Greene R, Byrd D, Brookland R. Cancer Staging Manual, 8th edn. American Joint Committee on Cancer. New York: Springer, 2017.
30 Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568–77.
31 Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer Lung Cancer Study Group. Ann Thorac Surg 1995;60:615–22; discussion 622–3.
32 Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd edn. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S–90S.
33 Predina JD, Kunkala M, Aliperti LA, Singhal AK, Singhal S. Sleeve lobectomy: current indications and future directions. Ann Thorac Cardiovasc Surg 2010;16:310–18.
34 Wu Y, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1–6.
35 Fernando HC, Landreneau RL, Mandrekar S, et al. Impact of brachytherapy on local recurrence after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small cell lung cancer (NSCLC). J Clin Oncol 2013;31:Abstract # 7502.
36 Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86.
37 Collaud S, Stahel R, Inci I, et al. Survival of patients treated surgically for synchronous single-organ metastatic NSCLC and advanced pathologic TN stage. Lung Cancer 2012;78:234–8.
38 Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6.
39 PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998;352:257–63.
40 Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 2006;24:2998–3006.
41 Curran WJ, Jr., Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–60.
42 Saunders M, Dische S, Barrett A, et al. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 1997;350:161–5.
43 Belani CP, Wang W, Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol 2005;23:3760–7.
44 Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: results on radiation dose in RTOG 0617. J Clin Oncol 2013;31:S7501.
45 Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 2004;59:828–36.
46 Non-small Cell Lung cancer. Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995;311:899–909.
47 Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190–6.
48 Ardizzoni A, Boni L, Tiseo M, et al. Cisplatinversus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99:847–57.
49 Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8.
50 Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advancedstage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51.
51 Patel JD, Socinski MA, Garon EB, et al. A Randomized, Open-Label, Phase III, Superiority Study of Pemetrexed(Pem) + Carboplatin(Cb) + Bevacizumab(Bev) Followed by Maintenance Pem + Bev versus Paclitaxel (Pac)+Cb+Bev Followed by Maintenance Bev in Patients with Stage IIIB or IV Non-Squamous Non-Small Cell Lung cancer. (NS-NSCLC). ASTRO. Chicago, 2012.
52 Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol 2012;30:2055–62.
53 Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432–40.
54 Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521–9.
55 Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354–62.
56 Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97.
57 Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32.
58 Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35.
59 Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39.
60 Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50.
61 Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Proc ESMO, 2016.
62 Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184–91.
63 Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50.
64 Manegold M, von Pawel J, Zatloukal P, et al. Randomised, double-blind multicentre phase III study of bevacizumab in combination with cisplatin and gemcitabine in chemotherapynaпve patients with advanced or recurrent non-squamous non-small cell lung cancer (NSCLC): BO17704. J Clin Oncol 2007;25:LBA7514.
65 Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008;26:60–5.
66 Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665–73.
67 Huang SF, Liu HP, Li LH, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res 2004;10:8195–203.
68 Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004;101:13306–11.
69 Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8.
70 Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46.
71 Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42.
72 Sequist LV, Yang JC, Yamamoto N, et al. Phase III Study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34.
73 Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73.
74 Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689–99.
75 Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFRexpressing advanced non-small-cell lung cancer. Ann Oncol 2008;19:362–9.
76 Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–74.
77 Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6.
78 Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–203.
79 Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004–12.
80 Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011–19.
81 Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94.
82 Kim D, Ahn M, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:7533.
83 Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77.
84 Mehra R, Camidge DR, Sharma S, et al. First-in-human phase I study of the ALK inhibitor LDK378 in advanced solid tumors. J Clin Oncol 2012;30:3007.
85 Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–42.
86 Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863–70.
87 Davies KD, Le AT, Theodoro MF, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res 2012;18:4570–9.
88 Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from wholegenome and transcriptome sequencing. Genome Res 2012;22:436–45.
89 Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med 2012;18:375–7.
90 Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med 2012;18:382–4.
91 Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378–81.
92 Planchard D, Mazieres J, Riely GJ, et al. Interim results of phase II study BRF113928 of dabrafenib in BRAF V600E mutation–positive non-small cell lung cancer (NSCLC) patients. J Clin Oncol 2013;31:8009.
93 Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984–93.
94 Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33.
95 Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570–7.
96 The Elderly Lung Cancer Vinorelbine Italian Study Group. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 1999;91:66–72.
97 Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-smallcell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079–88.
98 Lilenbaum R, Zukin M, Pereira JR, et al. A randomized phase III trial of single-agent pemetrexed (P) versus carboplatin and pemetrexed (CP) in patients with advanced non-small cell lung cancer (NSCLC) and performance status (PS) of 2. J Clin Oncol 2012;30:Abstract # 7506.
99 Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–9.
100 Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351–60.
101 Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbineplus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719–27.
102 Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589–97.
103 Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043–51.
104 Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692–9.
105 Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78–98.
106 O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7.
107 Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–71.
108 Le Pechoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003–08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467–74.
109 Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84.
110 Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78–84.