Introduction
Edema and lymphoedema are common in the palliative care setting, with many possible contributing factors. The aims of this chapter are to:
- Explore the pathophysiology, incidence, and presentation of oedema in a palliative care setting.
- Describe screening and assessment requirements for determining the presence, severity, and distress caused by oedema.
- Explain the conservative treatment options commonly used for oedema in a palliative care setting.
Pathophysiology of edema in palliative care setting
Swelling is a frequently reported symptom during the palliative care phase of many conditions. While the lymphatic form of swelling, lymphoedema, is commonly associated with cancer treatments, particularly breast cancer (Fig. 1), oedemas and lymphoedema can occur during the palliative care phase of many diseases including chronic renal and heart failure, end-stage liver disease, and neurological conditions such as Parkinson’s and chronic obstructive pulmonary disease, among others (Real et al. 2016). Patients with swelling may suffer from oedema, lymphoedema, or a mixed presentation (Real et al. 2016).
In non-palliative care patients, non-lymphatic oedema occurs as a result of change in the permeability of the capillary walls. As a consequence, a gradient change occurs between the hydrostatic pressures of the blood vessels and tissues. This results in extraneous fluid in the tissues (Real et al. 2016). In contrast, lymphoedema results due to disruption to lymphatic pathways caused by surgery or other treatments or traumas affecting the lymphatic pathways, the congenital absence or limited presentation of lymphatic pathways, or an overload of lymphatic fluid beyond the capacity of the lymphatic system (Real et al. 2016; Padera et al. 2016; Lawenda et al. 2009). Additional causes or contributing factors to the development of oedema and/or lymphoedema in a palliative care setting have been summarized by Real et al. (2016) and may include:
- Lymph node blockage and/or lymphadenopathy
- Abdominal involvement/blockage caused by tumors or ascites
- Medical causes such as hypoalbuminaemia (Bar-Sela et al. 2010), anemia, venous hypertension, cor pulmonale, and general deterioration
- Symptomatic or asymptomatic deep vein thrombosis (DVT)
- Dependency and/or immobility
- Organ failure
- Overhydration (Nwosu et al. 2016)
- Previous or ongoing treatments such as taxanebased chemotherapies or long-term steroid use
In palliative care, both forms of oedema are seen, often with the two forms combining (mixed oedema), resulting in possible significant swelling. A recent retrospective study found that 46% of oedemas were of the mixed variety, 29% were lymphoedema, and 10% were non-lymphaticrelated oedema (Real et al. 2016). While clearly there are many forms of swelling, the more general term oedema will be used from here on in this chapter to avoid confusion.
The incidence of oedema varies among studies and disease states. For example, various studies found that swelling occurs in 39% (Lau et al. 2010) to 73% (O’Connor and Kumar 2012) of chronic renal failure patients. This lack of agreement may be due, in part, to differences in diagnostic criteria for determining the presence of the oedema (Dylke et al. 2016). A conservative estimate would suggest that at least one-third of patients with cancer or noncancer end-stage conditions suffer from some form of oedema (Lau et al. 2010).
Edema may present in any region of the body during palliative care, although peripheral oedema is the most common presentation (Steindal et al. 2013). It is most commonly found in the lower limbs irrespective of the underlying disease state (Real et al. 2016), particularly in older patients (Steindal et al. 2013), possibly due to immobility and deconditioning. The location of oedema may also depend on the underlying condition that has led to the swelling. For example, secondary to breast cancer treatment, the oedema may present in the affected side’s upper limb, while secondary to oral cancers, the swelling may present in the head and neck region.
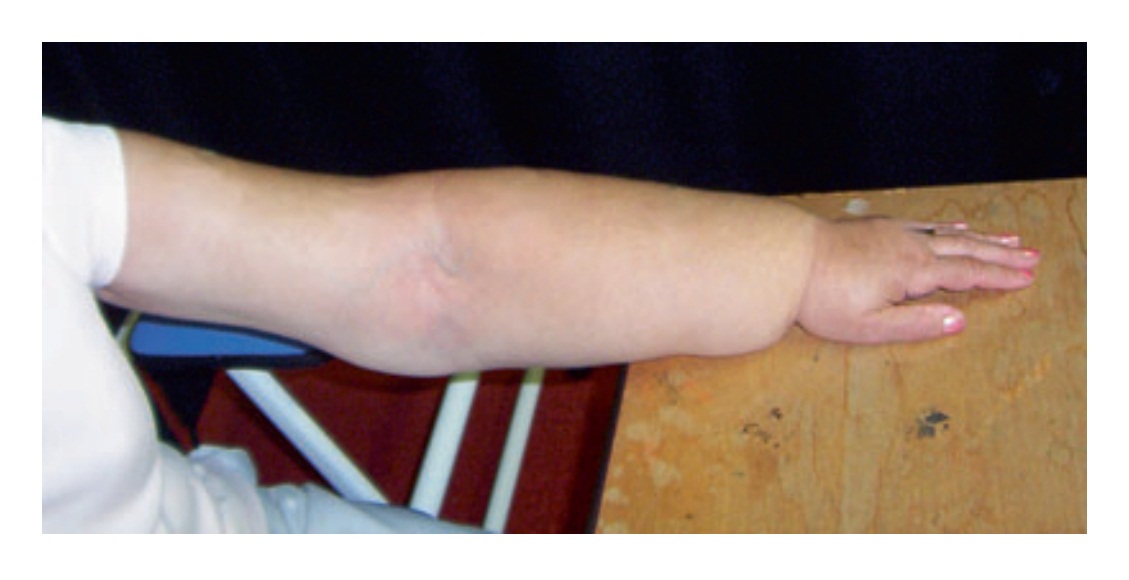
Fig. 1. Breast cancer patient with significant lymphoedema of the forearm
Edema presentation can change or progress (Lawenda et al. 2009). This progression may either be an increase in volume or change in tissue composition or a combination of both (Lawenda et al. 2009). When oedema first develops, the increase in volume of the swollen region is fluidbased, due to increase in either intracellular fluid, extracellular fluid, or a combination (International Lymphedema Framework and Canadian Lymphedema Framework 2010). For some, despite treatment, the volume of the limb may continue to increase, while for others the limb volume may stabilize (Dylke et al. 2013) and reduce in response to treatment (Ezzo et al. 2015). Beyond the increases in volume, particularly with oedema with a lymphatic contribution, the oedema may lead to changes in the tissue composition of the limb (Dylke et al. 2013). This change in tissue composition is not well understood but likely has multiple underlying causes including the high protein levels and inflammatory factors found in extracellular fluid (Padera et al. 2016). In the early stages, the high protein fluid in the limb leads to a presentation of pitting, where localized pressure leaves an indent in the tissues (Lawenda et al. 2009; International Lymphedema Framework and Canadian Lymphedema Framework 2010). With progression, pitting may cease and localized increases in fatty and fibrotic tissue occur (Dylke et al. 2013). These changes in limb composition are not simply a result of time with oedema and have been found in patients with both large and small limb volume increases (Dylke et al. 2013). As with increases in limb volume, it is currently unclear which patients are most at risk for changes in tissue composition.
Another possible consequence of oedema progression is skin changes. For some this will result in fibrotic, woody, or thickened skin (Padera et al. 2016; Lawenda et al. 2009), while others may experience fragile skin (International Lymphedema Framework and Canadian Lymphedema Framework 2010). Which presentation occurs may be due to the underlying condition causing the oedema, with heart and liver failure-related oedema as well as dependency oedema all being more likely to lead to fragile skin (International Lymphedema Framework and Canadian Lymphedema Framework 2010). Both thickened and fragile skin cause susceptibility to lesions or ulcers as well as skin infections (cellulitis) (Moffatt et al. 2003). Lymphorrhea, a leakage of lymphatic fluid through the edematous skin, may also occur with progressing oedema. Lymphorrhea also causes susceptibility to cellulitis and has been shown to be a poor prognostic indicator in a palliative care setting (Real et al. 2016).
Screening
Screening for oedema should be done systematically, through the use of targeted questioning or a questionnaire. Reliance on patients to volunteer that oedema is present likely leads to vast underdiagnosis of its presence as well as its impact. Homsi et al. (2006) found that without systematic questioning, only 4% of palliative care patients volunteered that they were experiencing swelling. However, the incidence rose to 30% of patients when they were systematically questioned about the presence of oedema, a finding also identified by others (White et al. 2009).
In addition to identifying more cases of oedema through systematic questioning or the use of a questionnaire, on average, eight more symptoms were identified than when a patient was left to self-report what they were experiencing (White et al. 2009). Importantly, the symptoms discovered on systematic questioning were often as severe in presentation and as distressing to the patient as those that were selfreported without prompting (Homsi et al. 2006). Notably, for oedema, the relationship between physical changes of oedema and level of distress was asynchronous, with small increases in limb volume associated with high levels of distress in some patients but not all (Homsi et al. 2006). Severity and distress caused by the oedema, therefore, should be assessed separately in determining the priorities for treatment.
Due to the commonality of oedema, a standardized approach to screening and onward referral for assessment of oedema is required. Without such processes in place, many of those with oedema are likely not detected and therefore not offered treatment (Thomson and Walker 2011). There is currently no agreed upon questionnaire nor screening method for detection of oedema in either the nonpalliative or palliative care setting. Commonly used, validated multi-symptom cancer assessment instruments such as the MD Anderson Symptom Inventory (Portenoy et al. 1994) and the Edmonton Symptom Assessment System (Bruera et al. 1991) do not include a question related to oedema. Of the small number of questionnaires validated in the palliative care setting that focus on symptom presence (Stromgren et al. 2002), only the Memorial Symptom Assessment Scale Short Form (Chang et al. 2000) specifically asks about swelling or oedema. This scale includes a single item, in which patients are asked whether or not oedema is present in either the arms or legs. If the patient indicates that swelling is present, further questioning of the patient occurs in regard to the severity and distress. However, a limitation of this scale is that it does not ask about swelling elsewhere, e.g., the head and neck or trunk. As the currently available validated questionnaires are not comprehensive in assessing all of the symptoms reported by patients and symptoms are not comprehensively recorded in patient records (Homsi et al. 2006), screening for oedema and likely other symptoms cannot rely on a single questionnaire; screening should be supplemented by questioning of the patient.
In the non-palliative care setting, the limb is also physically measured to detect the presence of swelling. Tools used to assess whether swelling is present in the “at-risk” limb include a tape measure to quantify limb circumference and/or used to derive limb volume, water displacement and Perometry to assess limb volume, and bioimpedance spectroscopy to assess extracellular fluid volume (of which lymph is a major contributor). The measurement methods for using these tools will be discussed further below.
Ideally, oedema is detected and treated when it is mild. However, one of the challenges for all oedemas has been the determination of criteria for its detection in a clinical environment. The lack of clear diagnostic criteria has resulted in the formation of a range of arbitrary thresholds (Dylke et al. 2016). This issue has been addressed for detection of mild upper limb lymphoedema arising from treatment for early breast cancer. Comparison of a range of commonly used and normatively based thresholds against a reference standard led to the identification of evidence-based diagnostic thresholds for detection of upper limb lymphoedema (Dylke et al. 2016). However, similar evidence-based diagnostic thresholds for other regions, such as the lower limbs, have not been developed for either a non-palliative or a palliative care setting.
Comprehensive assessment
If swelling is likely present, it is important to determine the underlying cause(s) as this will inform treatment. While imaging methods such as lymphoscintigraphy, magnetic resonance lymphography, or indocyanine green lymphography can provide additional information about the underlying morphology of the lymphatic system (Munn and Padera 2014), they are rarely undertaken in a palliative care setting unless they will alter management strategies (Real et al. 2016). The focus, instead, is on a thorough clinical assessment of the areas with or at risk for swelling. As there are many factors that may contribute to both the oedema and the treatment decisions associated with assessment findings, only those with advanced training in oedema should undertake both the assessment and treatment of oedema.
The clinical assessment for oedema is multifaceted. As part of any standard assessment, the history related to the oedema, treatments received, and any benefit they received from the treatment in controlling the swelling and symptoms is obtained. A review of the medications prescribed may be useful, as a number of common medications, such as nonsteroidal anti-inflammatories (NSAIDs), may contribute to the development or severity of the oedema (International Lymphedema Framework and Canadian Lymphedema Framework 2010). Importantly, the patient is asked whether they are experiencing any symptoms in relation to the oedema and, if present, the level of distress associated with the symptoms and swelling. Typical symptoms of oedema may include sensations of heaviness, achiness, discomfort, or fullness (Lawenda et al. 2009). In addition, patients are asked about the extent to which the oedema impacts on their physical abilities.
Visual inspection and palpation of the region with oedema will identify issues that could impact on treatment (Lawenda et al. 2009). The region should be inspected for the presence of wounds, skin breakdowns, signs of venous insufficiency, skin infection, and lymphorrhea, all of which may contraindicate certain treatments for the oedema and/or require modification of other treatments. Signs indicative of venous insufficiency include the presence of varicose veins, difficultly locating ankle pulse, and pain or discomfort in the legs that is relieved when legs are elevated. Hands-on assessment will provide an indication as to the state of the tissues. The presence of pitting oedema, tested by the application of pressure for a specified amount of time (often 10–30 s) and then observing for the presence of a dent in the tissues once pressure is removed, provides an indication of oedema progression. In contrast, palpation of fibrosis or skin and tissue hardening indicates a later progression of oedema, which may respond better to different treatments than does pitting oedema.
Measurement of the limb size can be done to indicate the severity of the oedema and provide a benchmark against which response to treatment can be determined. However, for some patients, this may be too burdensome for the patient to undertake (International Lymphedema Framework and Canadian Lymphedema Framework 2010). Multiple assessment tools may be used, which one (or ones) selected depends on the availability of the tools and the location of the swelling. For upper and lower limb oedema, the most commonly used tool is a simple tape measure with which circumferential measurements are taken at regular intervals along a limb. When completed in a standardized manner, they have excellent reliability (Czerniec et al. 2010). If one limb is swollen, its circumference measurements are often compared to the unaffected limb to give an indication of extent and severity of oedema. For bilateral swelling where this is not possible, circumference measurements may be most useful to monitor the changes in the size as a response to treatment. Protocols have also been developed for the measurement with a tape measure of head and neck (Deng et al. 2016) and breast oedema (Kovacs et al. 2007). Circumference measurements may also be converted to a volume; however, this is mostly done in a research setting and unlikely to provide additional assessment or treatment guidance in a clinical environment. Other ways of measuring limb volume may include water displacement or Perometry, both of which are less commonly used in clinical settings.
If the oedema likely has a lymphatic component, bioimpedance spectroscopy (BIS) may provide an alternative for assessment of limb swelling, measuring the volume of extracellular fluid specifically. Surface electrodes are attached to extremities, and a harmless low-level current is passed though the region to determine the resistance. Each limb is assessed separately and an interlimb ratio determined. BIS has several advantages to other measures of limb volume, including less intrusive and being specific to extracellular fluid. Other measures used to assess limb volume, such as tape measurements, include fat, bone, muscle, and all fluids and therefore may miss small increases in extracellular fluid. Indeed, BIS is particularly well-suited to detection of mild oedema (Ward et al. 2008) but is also suited to monitoring change in oedema over time (Czerniec et al. 2016). When standardized protocols are used, BIS has excellent reliability (Czerniec et al. 2010). Currently standardized protocols are available for the measurement of unilateral and bilateral upper and lower limb lymphoedema (Ward et al. 2011a, b).
Further objective assessments may be required to ensure appropriate treatments are offered dependent on the region affected and additional symptoms being reported. These may include a measurement of range of motion or strength of both the area with swelling and the whole body, an assessment of speaking or swallowing, gait assessment, or a pain and/or neuropathic pain assessment. After completing the subjective and objective assessments, if questions remain about the contributing factors to the swelling, additional investigations may need to be undertaken to ensure the cause of the oedema is determined and appropriate treatment is given (Box 1).
Although the physical assessments used for oedema are well-described and reliable to perform, there are gaps. For example, it is unclear what are the expected responses to treatments for most forms of oedema. The measurements ascertained, therefore, need to be used in conjunction with the results from the additional investigation, as well as the patient’s self-report of the issues and clinical reasoning to guide the intensity, level, and types of treatments offered.
Treatment of lymphedema
To date, management of most oedemas is conservative or surgical and not with pharmaceutical approaches. Surgical approaches are rarely warranted in a palliative care setting. However, with better understanding of the underlying contributions to oedema, pharmaceutical approaches may become available. For other oedemas encountered in the palliative care setting, medical management (including pharmaceutical) in combination with conservative treatment such as the use of compression may be warranted.
Ideally, oedemas are detected when the condition is mild. At this phase, the impact on function is minimal; the aim of treatment, therefore, is to manage symptoms through reduction in swelling volume. Exercises and compression may be all that is required in the mild stage (International Lymphedema Framework 2006). Frequently, however, more comprehensive treatments are required.
Traditionally, conservative treatment for oedema is divided into two phases: (i) intensive phase in which the aim of treatment is to reduce the volume of the limb and improve its shape and (ii) maintenance phase in which the aim is to maintain the reduction (International Lymphedema Framework 2006). However, in the palliative care, it is recognized that these distinct phases are blurred (International Lymphedema Framework and Canadian Lymphedema Framework 2010), with treatment focused on reducing the burden to the patient in terms of both the impact of the disease and the treatment.
Complex decongestive therapy
The most common treatment advocated for oedema is complex physiotherapy treatment (a.k.a. complex decongestive therapy) (International Lymphedema Framework 2006). Complex decongestive therapy is multifocal and comprises the following components: (i) manual lymphatic drainage, (ii) compression therapy, (iii) skin care, (iv) education, and (iv) exercise. This approach is time-consuming and, in its fullness, may not be tolerated by patients in the palliative care setting (Cheville et al. 2014).
(i) Manual Lymphatic Drainage
Manual lymphatic drainage (MLD) is a specialized form of light massage designed to encourage the drainage of lymphatic fluid from the limb (International Lymphedema Framework 2006). It is performed by MLD therapists trained in the anatomy and physiology of the lymphatic system to facilitate lymph drainage of the vessels (Ezzo et al. 2015). It is proposed that MLD can “assist nature” by stimulating the natural peristaltic contractions of the lymphangions, reducing hydrostatic resistance to lymph flow, and rerouting lymph away from areas of stasis and into viable lymphatic vessels (Ezzo et al. 2015). The evidence supporting its use, however, is weak, particularly for upper limb lymphoedema secondary to breast cancer (Ezzo et al. 2015). However, synthesis of the data in a Cochrane review provided preliminary data in a secondary analysis that MLD may be particularly effective early in the development of oedema, where the lymphatic system is still functioning relatively well (McNeely et al. 2004). Consequently, massage would be capable of both stimulating lymphatic flow and rerouting lymphatic flow via collaterals. When oedema has progressed, MLD may not have a major impact on reduction in limb volume. The addition of MLD to compression in studies which included moderate to severe upper limb lymphoedema only reduced limb volume by 7% (Ezzo et al. 2015).
Box 1. Additional assessments may need to be undertaken if the cause of the swelling is unclear and/or to guide treatment decisions
| Assessment | Reason | Possible alternations to treatment decisions |
| Electrocardiogram (ECG) | Cardiac failure | Medical management including medications may be indicated Lower compression pressure may be indicated |
| Ankle-brachial pressure index (ABPI) | Venous insufficiency | An ABPI of less than 0.8, particularly in the presence of ulceration or wounds, suggests that high pressure bandaging is contraindicated |
| Blood tests | Albumin levels |
Albumin is a protein that assists in preventing fluid leakage into the tissues
Low levels of albumin (hypoalbuminemia) may be indicative of kidney or liver disease or the body not absorbing enough nutrients Further investigations and/or medical management of underlying condition required |
| Ultrasound | Deep vein thrombosis (DVT) |
Medical management may be indicated
Use of compression may need modification |
While MLD may not contribute to significant volume reduction, it does have additional benefits, particularly in the palliative setting. In a study investigating the effect of MLD on palliative patients, it was noted that MLD was tolerated by most patients, and it was associated with significant reduction in both pain and dyspnea above that received from administration of opioids in combination with analgesics and co-analgesics (Clemens et al. 2010). MLD is typically given in a quiet, calm environment; this form of massage is very gentle and soothing which may contribute to the improved dyspnea and reduced pain. In contrast to simple massage, manual lymphatic drainage is also associated with improved emotional function in terms of reducing worry, irritability, tension and feelings of depression, dyspnea, and reduced sleep disturbance (Williams et al. 2002). Thus, while MLD may not be effective in addressing the increase in limb volume, it does offer benefit to the patient in other domains.
Although MLD can be beneficial, there are instances when it is contraindicated. Particularly when swelling is present in the lower leg, it is important to first rule out the presence of deep vein thrombosis. If present, MLD to that region may be contraindicated.
(ii) Compression
Compression is considered a keystone for treatment of oedema. The mechanisms underpinning the impact of compression on the lymphatic and vascular system are currently unclear, particularly as tissue composition within the edematous limb changes over time. Studies, particularly in relation to compression on venous blood flow, suggest that the increase in local pressure obtained from compression limits capillary fluid filtration while improving lymphatic drainage. The increased tension on the anchoring filaments of the initial lymphatics contributes to the opening of these initial lymphatics. As the valves in these lymphatics are unidirectional, fluid is pulled in and toward the lymph collectors which will be pulled open. Although the mechanism underlying how it works is unclear, it does contribute to decrease in limb volume (Ezzo et al. 2015; Badger et al. 2004). Compression may be delivered through a range of products. Types of devices used to apply compression include short-stretch bandages, off-the-shelf or customized compression garments, Velcro wraps which are easy to don and doff, or the use of compression pumps.
Short-stretch bandaging is typically advocated as part of Phase 1 of complex decongestive therapy to reduce volume and improve the shape of the limb (International Lymphedema Framework 2012). For upper limb lymphoedema, compression levels are suggested in the range of 20–30 mmHg, whereas with lower limb lymphoedema, compression levels of 60 mmHg are recommended (Partsch et al. 2011). These bandages, in contrast to others such as traditional tensor or “ace” bandages, stretch less than 50% when extended longitudinally. The pressure mediated by the bandages can be controlled by the width and number of layers used in combination with the tension of the fabric (Partsch et al. 2008). Another advantage in the use of bandaging is that it can be used in combination with padding and “chip bags” to ensure pressure is not applied to bony areas and to help soften hard fibrotic regions. The traditionally used multilayer compression system for reduction of limb volume has some limitations: this form of bandaging is associated with inconsistency in application techniques resulting in inconsistent pressures, variable results, and bulkiness, which can impede patients from wearing normal footwear and clothing, leading to a low level of adherence and the potential risk of falling (Lamprou et al. 2011). Another problem can be slippage and bunching, leading to uneven distribution of compression, which can result in discomfort at night and the potential for skin breakdown.
New approaches to bandaging are being explored. For example, the two-component compression 3M Coban system is less bulky, with preliminary data suggesting that the findings are equally effective in both treatment of chronic venous insufficiency (Moffatt et al. 2008) and moderate to severe lower limb lymphoedema (Lamprou et al. 2011). Importantly, although not formally assessed, participants treated with the two-component compression system reported enhanced mobility and comfort during walking than with previously worn compression systems (Lamprou et al. 2011). The two-component Coban system comprises latex-free roll bandages in which the inner layer is polyurethane foam and the outer layer is a cohesive bandage. The foam replaces the padding used in traditional multilayer bandaging, protecting bony prominences and providing the necessary grip to prevent slippage. The cohesive outer surface enables the layers to bond to each other, providing a stiff stable layer with no internal movement (Lamprou et al. 2011; Moffatt et al. 2012). Rosidal Soft (Activa Healthcare Ltd) foam rolls have also been used to replace bulky wadding typically used with patients with lymphoedema. Following application of the foam, cohesive short-stretch bandages were applied in figure of eight techniques, leading to significant reduction in the volume of the affected leg after a course of 12 days of bandaging (Whitaker et al. 2015).
The evidence clearly supports bandaging to reduce limb volume in lymphoedema (Badger et al. 2004). However, the patient in palliative care may not tolerate this form of intervention, nor has its effectiveness been explored for other causes of oedema. Alternative methods of compression may need to be explored.
Compression garments are readily available. Depending on the severity and location of the swelling, garments may be off-the-shelf or custom-made. However, patients may not tolerate the sustained pressure or be able to complete the application and removal of the compression garment. Other factors may also interfere with the use of compression garments, such as fragile skin, which can be stressed with the use of compression garments, lymphorrhea, and open wounds. An alternative to compression garments are compression “wraps,” in which low-elastic material section wrap across the limb and are secured with Velcro (Williams 2016). The advantage with a wrap is that they are easy to put on and adjust the pressure. However, it was noted that there is difficulty in using wraps if the person is overweight or inflexible or if there were severe shape distortion of the limb (Mosti et al. 2015).
(iii) Exercise
Typically, as part of standard treatment for oedema, patients are encouraged to exercise (International Lymphedema Framework 2006). Depending on the individual’s ability in relation to disease status, exercises such as resistance training, stretching, and aerobic exercise may be beneficial. Exercises are typically performed while using a form of compression, e.g., compression garment. In non-palliative care patients, resistance training has been shown to be protective against exacerbations of lymphedema, and associated with reduced symptoms, and increased strength (Schmitz et al. 2009). However, at the palliative stage, vigorous exercises may not be feasible. Rather, the focus may be on functional activities, such as rising from a chair and walking or repetitive contractions and relaxation of muscle groups in the lymphedematous region (Cheville et al. 2014).
Alternative treatments for patients in palliative care
Subcutaneous drainage of lower limb oedema has been piloted in patients in palliative care due to advanced cancer (Bar-Sela et al. 2010; Jacobsen and Blinderman 2011). Patients with severe lymphoedema of the lower body and leg, in whom diuretics and other conservative approaches were not providing any benefit, were offered this novel treatment. As part of the informed consent, patients were explicitly informed of the lack of supporting evidence for this treatment as well as the potential side effects of treatment (Bar-Sela et al. 2010). This approach evolved, from inserting subcutaneous catheters into the medial and lateral sides of the ankle and then connecting the catheter via tubing to an enclosed drainage baggage to creating subcutaneous tracts with needles in both ankles and following removal of the needles, wrapping the region with absorbent pads (Bar-Sela et al. 2010). The most recent modification was to remove both the needles and padding and let the region drain into a bucket. Five of eight patients who underwent this procedure identified improvement in mobilization that impacted their quality of life (Bar-Sela et al. 2010).
There are currently no medications available for the prevention or treatment of most forms of oedema (International Lymphedema Framework 2006). In a non-palliative care setting, diuretics have been shown to be ineffective in the management of lymphoedema (Kligman et al. 2004). However, in other forms of oedema, particularly if the peripheral oedema is a result of congestive heart failure, diuretic therapy can help reduce the severity of the swelling (Brake and Jones 2017). The dosage of diuretics given may need up or down titration depending on various factors and needs to be done in the context of the wider symptoms and medical presentation (Brake and Jones 2017). Alternatively, as some medications, such as methadone for pain, may cause or worsen oedema, a review of the medications being taken by the patient may lead to improvements in symptoms (International Lymphedema Framework and Canadian Lymphedema Framework 2010; Dawson et al. 2014).
Hydration levels may also impact on oedema severity, with patients with higher hydration levels, suffering from worse oedema (Nwosu et al. 2016). However, following guideline-based artificial hydration therapy protocols has been shown to lead to a significant improvement in oedema (Nakajima et al. 2014).
Conclusion and summary
Edema in a palliative care setting is a common, complicated, multifaceted, and often distressing side effect of the end stage of many diseases. It can have numerous contributing factors, thereby requiring thorough screening and assessment to ensure the underlying causes are known and appropriate treatments are offered. Treatment of oedema in a palliative care setting has been shown to improve quality of life and mobility and reduce other symptoms such as pain and dyspnea. While there are a range of treatments available, in a palliative care setting, the focus is often on managing the symptoms and distress caused by the oedema rather than reducing the volume of the limb. This often results in necessary modifications to the approach and intensity of the treatment. A growing understanding of the lymphatic system and its role in fluid management may lead to new treatments in the future.
References
Badger C, Preston N, Seers K, Mortimer P. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev. 2004;(4): Cd003141.
Bar-Sela G, Omer A, Flechter E, Zalman D. Treatment of lower extremity edema by subcutaneous drainage in palliative care of advanced cancer patients. Am J Hosp Palliat Care. 2010;27(4):272–5.
Brake R, Jones ID. Chronic heart failure part 2: treatment and management. Nurs Stand. 2017;31(20):53–63.
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9.
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The memorial symptom assessment scale short form (MSAS-SF). Cancer. 2000;89(5):1162–71.
Cheville AL, Andrews K, Kollasch J, Schmidt K, Basford J. Adapting lymphedema treatment to the palliative setting. Am J Hosp Palliat Care. 2014;31(1):38–44.
Clemens KE, Jaspers B, Klaschik E, Nieland P. Evaluation of the clinical effectiveness of physiotherapeutic management of lymphoedema in palliative care patients. Jpn J Clin Oncol. 2010;40(11):1068–72.
Czerniec SA, Ward LC, Refshauge KM, Beith J, Lee MJ, York S, et al. Assessment of breast cancer-related arm lymphedema – comparison of physical measurement methods and self-report. Cancer Investig. 2010;28 (1):54–62.
Czerniec SA, Ward LC, Kilbreath SL. Breast cancerrelated arm lymphedema: fluctuation over six months and the effect of the weather. Lymphat Res Biol. 2016;14(3):148–55.
Dawson C, Paterson F, McFatter F, Buchanan D. Methadone and oedema in the palliative care setting: a case report and review of the literature. Scott Med J. 2014;59(2):e11–3.
Deng J, Dietrich MS, Ridner SH, Fleischer AC, Wells N, Murphy BA. Preliminary evaluation of reliability and validity of head and neck external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2016;22:63–70.
Dylke ES, Ward LC, Meerkin JD, Nery L, Kilbreath SL. Tissue composition changes and secondary lymphedema. Lymphat Res Biol. 2013;11(4):211–8.
Dylke ES, Schembri GP, Bailey DL, Bailey E, Ward LC, Refshauge K, et al. Diagnosis of upper limb lymphoedema: development of an evidence-based approach. Acta Oncol. 2016;55:1477–83.
Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. 2015;(5):Cd003475.
Homsi J, Walsh D, Rivera N, Rybicki LA, Nelson KA, Legrand SB, et al. Symptom evaluation in palliative medicine: patient report vs systematic assessment. Support Care Cancer. 2006;14(5):444–53.
International Lymphedema Framework. International Consensus: Best Practice for the Management of Lymphedema: www.lympho.org; 2006. Available from: http:// www.woundsinternational.com/media/issues/210/files/ content_175.pdf
International Lymphedema Framework. Compression Therapy: A position document on compression bandaging. Best Practice For the Managament of Lymphedema. 2nd ed. [Internet]. 2012. Available from: http:// www.lympho.org/mod_turbolead/upload//file/ Resources/Compression%20bandaging%20-%20final. pdf. 12 Apr 2017.
International Lymphedema Framework and Canadian Lymphedema Framework. The management of lymphoedema in advanced cancer and oedema at the end of life. International Lymphedema Framework Postiion Statement [Internet]. 2010. Available from: http://www.lympho.org/portfolio/the-management-oflymphoedema-in-advanced-cancer-and-oedema-at-the-end-of-life/. 12 Apr 2017.
Jacobsen J, Blinderman CD. Subcutaneous lymphatic drainage (lymphcentesis) for palliation of severe refractory lymphedema in cancer patients. J Pain Symptom Manag. 2011;41(6):1094–7.
Kligman L, Wong RK, Johnston M, Laetsch NS. The treatment of lymphedema related to breast cancer: a systematic review and evidence summary. Support Care Cancer. 2004;12(6):421–31.
Kovacs L, Eder M, Hollweck R, Zimmermann A, Settles M, Schneider A, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast (Edinburgh, Scotland). 2007;16(2):137–45.
Lamprou DA, Damstra RJ, Partsch H. Prospective, randomized, controlled trial comparing a new two-component compression system with inelastic multicomponent compression bandages in the treatment of leg lymphedema. Dermatol Surg. 2011;37 (7):985–91.
Lau KS, Tse DM, Tsan Chen TW, Lam PT, Lam WM, Chan KS. Comparing noncancer and cancer deaths in Hong Kong: a retrospective review. J Pain Symptom Manag. 2010;40(5):704–14.
Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin. 2009;59(1):8–24.
McNeely ML, Magee DJ, Lees AW, Bagnall KM, Haykowsky M, Hanson J. The addition of manual lymph drainage to compression therapy for breast cancer related lymphedema: a randomized controlled trial. Breast Cancer Res Treat. 2004;86(2):95–106.
Moffatt CJ, Franks PJ, Doherty DC, Williams AF, Badger C, Jeffs E, et al. Lymphedema: an underestimated health problem. QJM. 2003;96(10):731–8.
Moffatt CJ, Edwards L, Collier M, Treadwell T, Miller M, Shafer L, et al. A randomised controlled 8-week crossover clinical evaluation of the 3M Coban 2 Layer Compression System versus Profore to evaluate the product performance in patients with venous leg ulcers. Int Wound J. 2008;5(2):267–79.
Moffatt CJ, Franks PJ, Hardy D, Lewis M, Parker V, Feldman JL. A preliminary randomized controlled study to determine the application frequency of a new lymphoedema bandaging system. Br J Dermatol. 2012;166(3):624–32.
Mosti G, Cavezzi A, Partsch H, Urso S, Campana F. Adjustable velcro compression devices are more effective than inelastic bandages in reducing venous edema in the initial treatment phase: a randomized controlled trial. Eur J Vasc Endovasc Surg. 2015;50(3):368–74.
Munn LL, Padera TP. Imaging the lymphatic system. Microvasc Res. 2014;96:55–63.
Nakajima N, Takahashi Y, Ishitani K. The volume of hydration in terminally ill cancer patients with hydration-related symptoms: a prospective study. J Palliat Med. 2014;17(9):1037–41.
Nwosu AC, Morris L, Mayland C, Mason S, Pettitt A, Ellershaw J. Longitudinal bioimpedance assessments to evaluate hydration in POEMS syndrome. BMJ Support Palliat Care. 2016;6(3):369–72.
O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012;15(2):228–35.
Padera TP, Meijer EF, Munn LL. The lymphatic system in disease processes and cancer progression. Annu Rev Biomed Eng. 2016;18:125–58.
Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27 (3):193–219.
Partsch H, Damstra RJ, Mosti G. Dose finding for an optimal compression pressure to reduce chronic edema of the extremities. Int Angiol. 2011;30 (6):527–33.
Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30a(9):1326–36.
Real S, Cobbe S, Slattery S. Palliative care edema: patient population, causal factors, and types of edema referred to a specialist palliative care edema service. J Palliat Med. 2016;19(7):771–7.
Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–73.
Steindal SA, Ranhoff AH, Bredal IS, Sorbye LW, Lerdal A. Last three days of life in the hospital: a comparison of symptoms, signs and treatments in the young old and the oldest old patients using the Resident assessment instrument for palliative care. Int J Older People Nursing. 2013;8(3):199–206.
Stromgren AS, Groenvold M, Pedersen L, Olsen AK, Sjogren P. Symptomatology of cancer patients in palliative care: content validation of self-assessment questionnaires against medical records. Eur J Cancer. 2002;38(6):788–94.
Thomson M, Walker J. Collaborative lymphoedema management: developing a clinical protocol. Int J Palliat Nurs. 2011;17(5):231–8.
Ward L, Kilbreath S, Cornish B. Bioimpedance analysis for early detection of lymphedema. In: Weissieder H, Schuchhardt C, editors. Lymphedema: diagnosis and therapy. 4th ed. Essen: Viavital Verlag; 2008. p. 502–17.
Ward L, Winall A, Isenring E, Hills A, Czerniec S, Dylke E, et al. Assessment of bilateral limb lymphedema by bioelectrical impedance spectroscopy. Int J Gynecol Cancer. 2011a;21(2):409–18.
Ward LC, Dylke E, Czerniec S, Isenring E, Kilbreath SL. Reference ranges for assessment of unilateral lymphedema in legs by bioelectrical impedance spectroscopy. Lymphat Res Biol. 2011b;9(1):43–6.
Whitaker J, Williams A, Pope D, Elwell R, Thomas M, Charles H, et al. Clinical audit of a lymphoedema bandaging system: a foam roll and cohesive short stretch bandages. J Wound Care. 2015;24(3):83–4; 6–90; 2–4
White C, McMullan D, Doyle J. “Now that you mention it, doctor …”: symptom reporting and the need for systematic questioning in a specialist palliative care unit. J Palliat Med. 2009;12(5):447–50.
Williams A. A review of the evidence for adjustable compression wrap devices. J Wound Care. 2016;25 (5):242–7.
Williams AF, Vadgama A, Franks PJ, Mortimer PS. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancerrelated lymphoedema. Eur J Cancer Care. 2002;11 (4):254–61.