Introduction
Fatigue is part of a regulatory system that serves to keep the balance between activities and rest. Unfortunately, patients who are suffering from life-limiting disorders tend to experience fatigue with an overwhelming intensity, leading to a state of imbalance where sleep and rest do not lead to recuperation. This combination of physical, emotional, and cognitive weariness or exhaustion is termed fatigue. The exact causes and mechanisms are not yet well understood, with no definitive diagnostic criteria or lab markers available, making fatigue difficult to recognize and properly diagnose. A thorough description of the symptoms ailing the patient is of utmost importance in the clinical setting. What is known to date is that in most cases, fatigue is not attributable to one single cause; it has a multifactorial genesis and is more than just the sum of its causes. It is an ailment with multiple layers which severely limits the patient’s quality of life during their disease trajectory. The vast majority of palliative care patients are subjectively affected by this complex symptom. Fatigue in patients with life-limiting diseases reduces, alone or in combination with other symptoms such as pain, dyspnea, or nausea, the overall quality of life considerably and plays a significant role as one of the most important prognostic factors. Fatigue can manifest itself at any time in the course of a life-limiting disease and can even be observed many years after cancer therapy. Data regarding the prevalence of fatigue varies tremendously depending on the clinical definition of fatigue, diagnostic tools, the form of therapy, and the point in time during which examinations are carried out. The clinical appearance of fatigue has many faces and includes physical, cognitive, and affective aspects. Problems such as the multidimensional clinical appearance of fatigue and the lack of a unifying model explaining its etiology and pathogenesis make it most difficult to formulate a universal definition of this syndrome.
The identification and treatment of fatigue is an interdisciplinary task which involves all parts of the health care team. In the last few years, there has been enormous growth in literature regarding clinical experience with fatigue, as well as fundamental research into the mechanisms of this syndrome. This chapter is geared toward all professional health care groups which provide care to palliative care patients. By discussing topics such as the clinical manifestation of fatigue, etiology, leading theories and promising research, diagnostics, management, non-pharmaceutical therapies, pharmaceutical therapies, and rehabilitation, this chapter aims to provide an overview but also highlights how much is left to research and how little we understand this prevalent syndrome. We hope that this chapter will aid our colleagues in the health care field to better recognize, understand, and effectively treat this very complex ailment.
Overview
Fatigue is defined by the EAPC (European Association of Palliative Care) as a subjective feeling of tiredness, weakness, or lack of energy (Radbruch et al. 2008). It can be thought of as a cluster of symptoms affecting the physical and cognitive capabilities of a person. These two aspects can then be further generalized as weakness representing the physical part and fatigue/ weariness representing the cognitive aspect. Approximately up to 80% of advanced stage cancer patients suffer from fatigue, making it next to pain and cachexia one of the most common symptoms of late-stage cancer. Fatigue can affect people of all ages, although there is currently in sufficient research regarding children and adolescents. Unlike healthy individuals, who recover their energy during rest and sleep, cancer patients suffering from fatigue do not, leading to a feeling of a heavy continuous strain and disability, as well as a lower perceived quality of life. Glaus, a leading expert on fatigue, stresses the importance of the qualitative differences between general fatigue that healthy people may experience and cancer-related fatigue (CFR), the latter which affects the long-term physical, affective, and cognitive aspects of the patient’s well-being. However, other authors have stressed that differences between general fatigue seen in healthy people and fatigue in palliative care patients may be due to the more overwhelming intensity of fatigue in palliative care patients rather than the qualitative difference between cancer patients.
The pathophysiology is not yet fully understood; this makes it difficult to suggest a broad general therapeutic approach. Fatigue is a multidimensional condition which requires a multimodal therapy. This includes physical, psychiatric, social, and spiritual interventions, which together can lead to an improved disease management and to a better quality of life.
Definition and prevalence
Multiple definitions have been suggested for the tumor-associated fatigue, also referred to as “cancer-related fatigue.” David Cella, who was the first to define fatigue in 1995, defined it as “a subjective state of overwhelming, sustained exhaustion and decreased capacity for physical and mental work that is not relieved by rest” (Cella et al. 1998). Over time, criticism emerged regarding the seemingly arbitrarily chosen duration and intensity of the symptoms. A consensus was reached when the Fatigue Coalition USA suggested to use the ICD-10 criteria, shown in Table 1 (Cella et al. 2001).
The National Comprehensive Cancer Network (NCCN) defined fatigue as follows: “Cancerrelated fatigue is a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (Berger et al. 2015).
The EAPC definition considers the physical and cognitive dimensions of fatigue as the least common denominator in their discussion, regarding the affective attributes as part of a natural consequence of the reduced quality of life. On the other hand, other definitions have highlighted additional dimensions of fatigue. Glaus, for example, described fatigue as a multidimensional symptom complex, which consisted of 59% physical, 29% affective, and 12% cognitive sensations (Glaus 1998). The physical symptoms are made up of decreased performance, weakness and lack of strength, extreme physical exhaustion, as well as an unusually increased need for sleep and rest. The affective and emotional level is comprised of a sense of helplessness, irritability, limited participation in usual activities, sadness, fear, and lethargy. The cognitive symptoms consist of difficulty concentrating, thought disorders, as well as sleep problems (falling asleep, staying asleep).
Table 1. Proposed ICD criteria for CFR
| Six (or more) of the following symptoms have been present every day or nearly every day during the same 2-week period in the past month, and at least one of the symptoms is significant fatigue (A1) | |
| A1 | Significant fatigue, diminished energy, or increased need to rest, disproportionate to any recent change in activity level |
| A2 | Complaints of generalized weakness or limb heaviness |
| A3 | Diminished concentration or attention |
| A4 | Decreased motivation or interest to engage in usual activities |
| A5 | Insomnia or hypersomnia |
| A6 | Experience of sleep as unrefreshing or nonrestorative |
| A7 | Perceived need to struggle to overcome inactivity |
| A8 | Marked emotional reactivity (e.g., sadness, frustration, or irritability) to feeling fatigued |
| A9 | Difficulty completing daily tasks attributed to feeling fatigued |
| A10 | Perceived problems with short-term memory |
| A11 | Post-exertional malaise lasting several hours |
| B | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning |
| C | There is evidence from the history, physical examination, or laboratory findings that the symptoms are a consequence of cancer or cancer therapy |
| D | The symptoms are not primarily a consequence of comorbid psychiatric disorders such as major depression, somatization disorder, somatoform disorder, or delirium |
Research has shown that fatigue is the most common and most debilitating of the problems faced by tumor patients after the conclusion of cancer therapy (Lawrence et al. 2004; Stark et al. 2012). Symptoms such as lack of energy and drowsiness have been observed in 74% and 60% of tumor patients, respectively. Around 30–50% of these patients reported undiminished fatigue even years after successful cancer treatment. The chances of suffering from fatigue rise to nearly 99% after chemoor radiotherapy, making fatigue one of the most debilitating side effects of these therapies. Not only cancer patients are plagued by this syndrome, patients suffering from other chronic life-threatening illnesses such as congestive heart failure, respiratory failure from COPD or lung fibrosis, or HIV/AIDS have a high chance of being afflicted by a significant fatigue symptom burden. For multiple sclerosis, fatigue is reported as the most common symptom with 83% of the patients being affected. In contrast to diseaserelated fatigue, the long-lasting chronic fatigue syndrome (CFS) can be observed in 0.3% of the population. Seventy percent of the CFS cases develop after an infection, so that an immune disorder or viral component is being hypothesized. The proposed diagnostic criteria are made up of three main criteria: a substantial reduction or impairment in the ability to engage in pre-illness levels of occupational, educational, social, or personal activities that persist for more than 6 months and are accompanied by fatigue, which is often profound, is of new or definite onset (not lifelong), is not the result of ongoing excessive exertion, and is not substantially alleviated by rest; postexertional malaise; and unrefreshing sleep (Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, and Institute of Medicine 2015). A psychological component is found in 40% of CFS patients.
Fatigue in palliative care patients must be differentiated from cachexia, or the anorexiacachexia syndrome (ACS).
Fearon et al. defined cachexia as:
«.. . a multifactorial syndrome characterised by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment. The pathophysiology is characterised by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism». (Fearon et al. 2011)
Further diagnostic criteria for cachexia include weight loss of over 2% over 2 months, more than 5% over 6 months, or a body mass index (BMI) less than 20 kg/m2. Muscle mass and stomach fat are not the only tissues affected by cachexia: atrophies and loss of functionality are seen in other systems, and until ultimately, heart failure leads to death.
Potential causes of fatigue
Patients presenting with fatigue should be checked for treatable causes of the symptoms. For example, depression or side effects from medications can mimic fatigue and should be excluded before the diagnosis of fatigue is made. Depression can be difficult to distinguish from fatigue due to overlapping symptoms such as weakness and lack of energy. Symptoms such as recurrent feelings of worthlessness or recurrent thoughts about death can be an indication for depression rather than fatigue. A list of potential causes of fatigue can be found in Fig. 1.
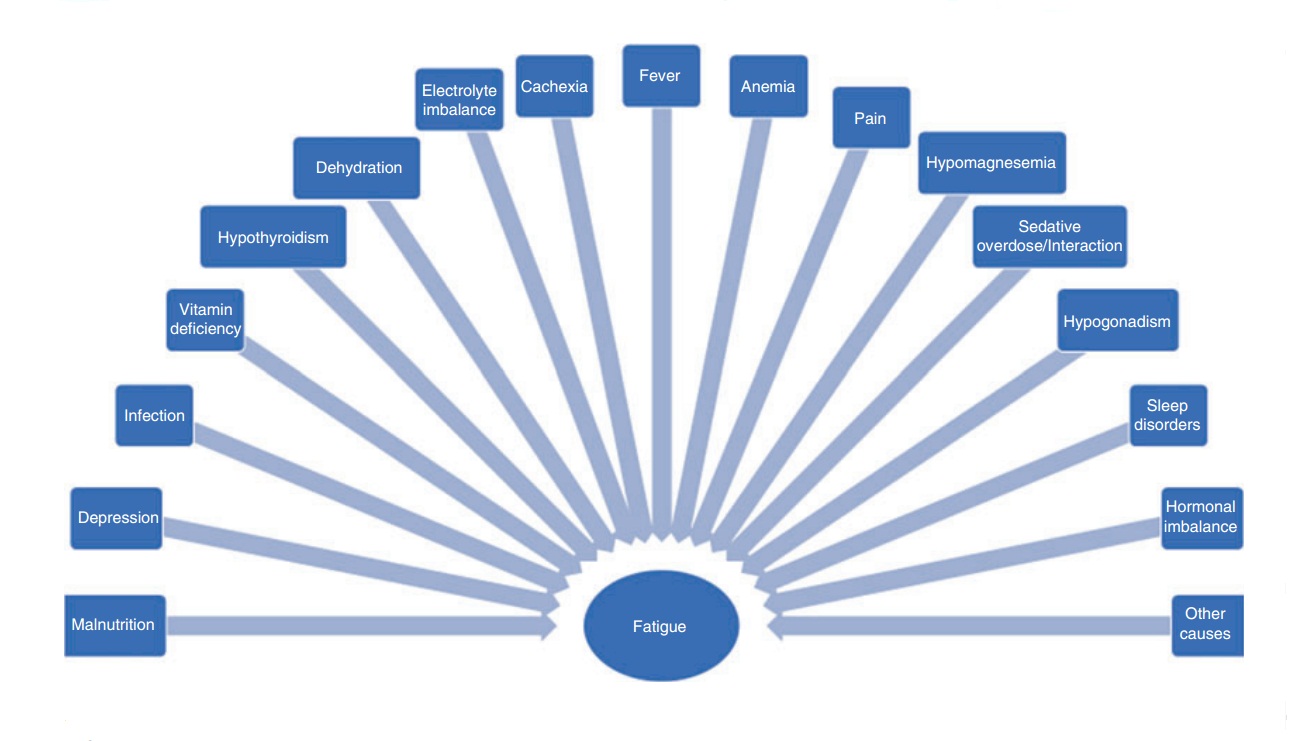
Fig. 1. Possible disorders leading to fatigue
Etiology and pathophysiology
To date, there exists no unifying theory which explains the etiology and pathogenesis of fatigue. Although, multiple different pathophysiological pathways have been described, it is widely believed that the origin of fatigue can be categorized as either peripheral or central. Peripheral fatigue has been associated with changes in muscle metabolism, while central fatigue has been related to a disorder in the hypothalamic-pituitaryadrenal (HPA) axis as well as the circadian rhythm, resulting in cognitive disabilities (Stone and Minton 2008; Wang 2008). It is possible that the various causes of fatigue require specific therapies and management. The etiology of cancerrelated fatigue (CRF) has not been yet fully explained. Cancer-associated symptoms, including CRF, are influenced by polymorphisms. For example, polymorphisms of genes regulating inflammatory cytokines were shown to be risk factors for cancer-related fatigue (Bower and Lamkin 2013; Collado-Hidalgo et al. 2008). Because of this, a multifactorial pathophysiology is presumed. The development of CRF can either be due to direct influences of the cancer or can result as a side effect of antineoplastic radioor chemotherapy. Radioor chemotherapy may also cause other side effects such as paraneoplasias, anemia, metabolic disorders, or cachexia, all of which may aggravate CRF.
Cancer-related fatigue may also be classified into primary and secondary. Primary CRF is thought to be directly related to the tumor itself, while secondary CRF is the result of physical distress related to pain, sleeping disorders, infections, malnourishment, hypothyroidism, and anemia, but also emotional stress and depression. Many factors are likely to contribute to CRF in late-stage cancer, so that a clear distinction between primary and secondary is not always possible (Radbruch et al. 2008). The use of pharmaceuticals can also contribute to the development of fatigue. For example, the use of opioids can lead to an opioid-induced fatigue due to their sedative attributes.
Due to our poor understanding of the pathophysiology of fatigue in palliative care patients, there is a pronounced need for more research. A few talking points which are currently being discussed as contributing or causal factors are:
- An increased production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 either by the tumor, as a reaction of the immune system, or by the paraneoplastic entity.
- A high serotonin level due to a dysregulation favors a reduction in the somatomotor drive.
- Activation of afferent vagal nerves by released neuroactive substances leads to suppression of somatic muscle activity and induction of weakness and weariness.
- Disruptions in the hypothalamic-pituitaryadrenal (HPA) axis pathway through cytokines, IFN-a, or IL-2 lead to ACTH (adrenocorticotropin) suppression and consequently a reduction in cortisol secretion.
- ATP (adenosine triphosphate) dysregulation in muscle cells due to a defect in ATP regeneration, muscle mass loss caused by cortisone intake, or certain chemotherapeutic substances.
- Disruption of the circadian rhythm and melatonin secretion. These hypotheses are partly based on studies that focused on illnesses characterized by the occurrence of fatigue, such as work-induced tiredness, CFS, and rheumatoid arthritis.
The patient’s perception
Most patients view CRF as part of an inevitable progression of their disease, which results in them rarely addressing it in conversation with their physicians. This may be reinforced by the patient’s assumption that there is nothing that can be done to alleviate CRF or that CRF treatment could have unwanted side effects. If the maintenance of physical performance is an important part of the patient’s self-perception, it may be difficult for them to admit a weakened resilience. In oncology, medical staff tend to put the emphasis of care on the main illness, leading to fatigue being perceived as a symptom of secondary importance which may distract from the primary goal of treating the cancer. A structured assessment with the help of questionnaires like the Edmonton Symptom Assessment System (ESAS) or the “minimal documentation system” (MIDOS) is helpful for the screening of relevant symptoms in clinical practice. The MIDOS2 is a
German adaptation of the ESAS and functions as a tool for repeated symptom self-assessments by the patients. It tests ten items (with two additional items being labeled as “other” which the patient can specify) on a four-step visual rating scale ranging from “not present,” “light,” “middle,” and “strong.” The ten items include pain, nausea, vomiting, dyspnea, constipation, weakness, lack of appetite, tiredness, depressive mood, and fear. In addition, the current state of well-being is also assessed (very bad, bad, moderate, good, and very good). Screening instruments such as the ESAS or MIDOS are easy to use and require little time to fill out, resulting in a lower burden for the patient but allowing a good overview of the patient’s situation for the medical staff (Stiel et al. 2010).
Fatigue and depression
It is not uncommon to see depression being associated with CFS. Differentiation between depression and fatigue is difficult, as there is considerable overlap in the diagnostic criteria. This becomes apparent when comparing the ICD criteria for depression with those for fatigue. For example, weariness is a pronounced symptom of depression, while sadness, fear, and lethargy have been described as part of an affective dimension of fatigue. Depression may aggravate the perceived burden of fatigue and the subjective suffering of the patient. Vice versa, it has been shown that fatigue is able to induce and intensify depression. However, differentiating between which parts of the symptom burden are attributable to fatigue and which to depression, and if predominantly one or both are contributing, may be important for the therapeutic regimen. In some cases, depression may be easier to treat than fatigue, and the successful treatment of depression may partly or even completely alleviate the fatigue symptomatic. When considering differential diagnoses, keep in mind that feelings of guilt, selfdeprecation, and a sense of imminent death are more typical of depression rather than fatigue. The differentiation between depression and fatigue is not always possible, even a skilled psychiatric specialist utilizing a structured clinical interview will sometimes not be able to distinguish the two. The patient history can be a helpful tool for separating the two syndromes if it includes questions regarding previous depressive episodes, if the symptoms of fatigue preceded the depressive symptoms, and whether the fatigue symptomatic is a new experience which occurred parallel to the underlying disease.
Fatigue and the social sphere
Fatigue is not just distressing for the affected patient, but is also a challenge for the whole surrounding social system, including the partner, family, and friends. The chronic form of fatigue, especially after long periods of therapy, often makes it difficult for patients to return to a daily routine. Friends and family have to realize that joint activities are no longer a trivial thing due to the lack of energy of the patient. Interpersonal roles, relationships, and social relations can change as a consequence of the illness. Friends might be reluctant to spend time together, as they feel overwhelmed by the condition of their friend and might not know how to react. This often leads to a feeling of disappointment and isolation by the patient and may in turn produce negative developments and setbacks in the course of the illness. Afflicted patients should be informed that their limitations due to exhaustion, their needs, and their expectations should be openly communicated within their social sphere. Only the greatest possible candor can minimize misunderstandings and help improve relationships. Professional help in the form of psychotherapists should also be offered early on to not only the patient but also to family and friends.
Fatigue when returning to work
For most patients, the ability to work is an integral part of their quality of life and strengthens their sense of self-reliance and purpose. Patients may be limited in their ability to work due to a lack of concentration, memory problems, and the reduced ability to think clearly, depending on the level of exhaustion. This can often lead to a delayed reintegration into their work life. Thus, patient information and education on topics such as acceptance of their illness, neuropsychological deficits, and the complex somatic limitations that go hand in hand with fatigue is of utmost importance. Rehabilitation assessments can offer a possibility to gauge the patient’s personal performance deficits, making it possible to arrange a personalized rehabilitation plan for the reintegration of the patient back into the workforce. An important aid for the return to work are stepwise reintegration programs where employee, employer, and physician agree on a step-by-step workload increase over a period of time until either a 100% workload or the highest possible workload that does not affect the patient’s recovery is reached. Unfortunately, this is not always feasible so that individual solutions must be found. An excessively demanding workload must be avoided as it can create recurring frustration and commonly ends in resignation which further amplifies the fatigue symptomatic. A similar strategy of openness and candidness as with family and friends is recommended in the work environment and with the employer. Problems should be addressed early on and openly to ?nd solutions that include, for example, reduction of work hours per week or transfer to a different workstation within the company if possible. Patients should take their time with tasks and be aware of their physical needs, taking special consideration as to possible signals of over-working. A detailed consultation with the health care provider team (physician, social worker, psychologist/psychiatrist) should take place to allow the planning of a personalized reintegration plan and offer a chance for the patient to ask questions, as well as considerations to be made for disease-related retirement.
Diagnostics
The goals of fatigue diagnostics in seriously ill patients are:
- To better understand the symptoms and problems ailing the patient
- To capture the “as is” status of the patient at that moment
- To aid in the structuring of their day-to-day life
- To be able to effectively prioritize energy reserves to complete vital tasks
- To document the successes, but also failures, of therapeutic options
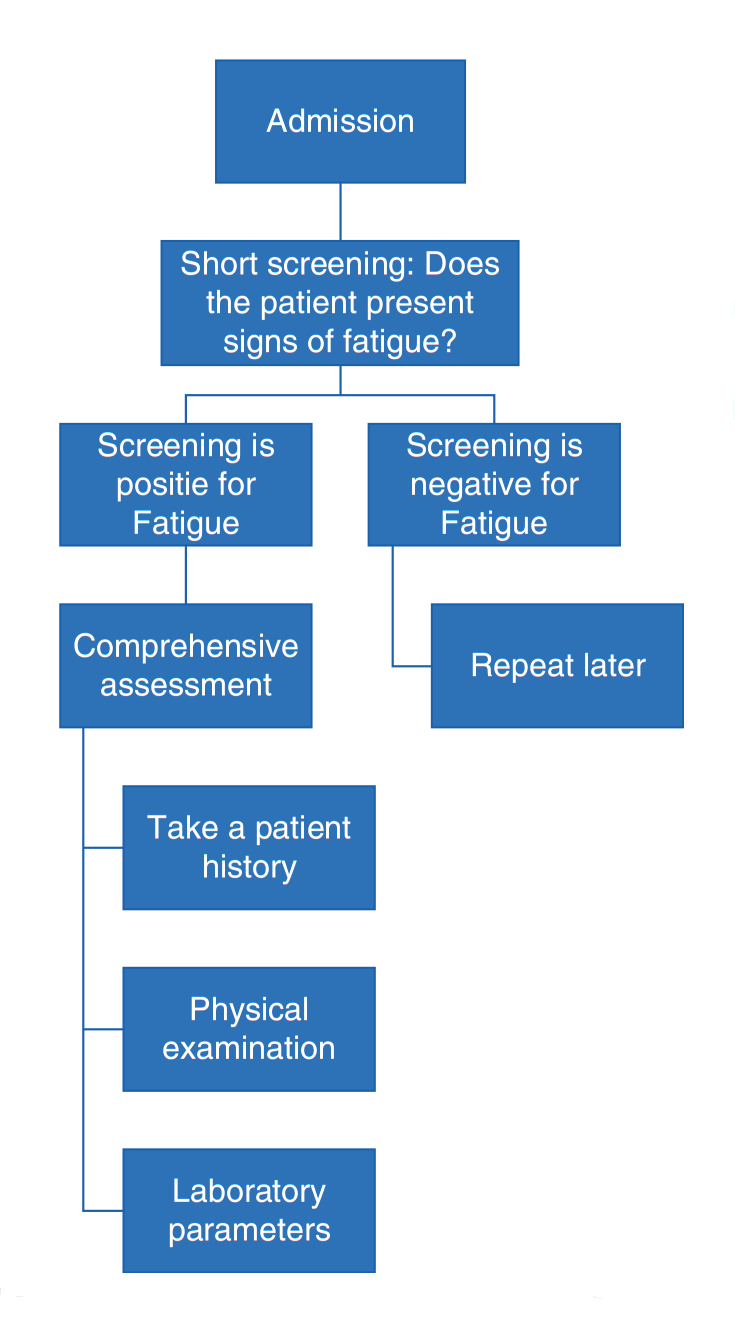
Fig. 2. Fatigue and weakness assessment on admission
Screening should include explicit questions regarding unusual tiredness or exhaustion as part of the patient’s history, as there are no reliable lab parameters or physical tests for fatigue. Answers to these screening questions will always be subjective, but self-assessment is the cornerstone of the fatigue diagnosis. Rating fatigue intensity on categorical, numerical, or visual analog scales, akin to the ones used to assess pain, can be useful to gauge the progression of the syndrome and the impact of therapeutic interventions. A differentiated and detailed fatigue and performance assessment also includes social anamnesis, physical activities and limits, and sleeping habits (Fig. 2).
In addition to the patient history, physical examination, and routine lab parameters, specific fatigue questionnaires are an effective way to round up the assessment. Some of the most commonly used quality-of-life questionnaires include the Short Form-36 Health Survey (SF-36) and the Quality of Life Questionnaire-C30 (QLQ) from the European Organisation for Research and Treatment of Cancer (EORTC), the latter being the official standard to assess quality of life in oncology which includes items regarding fatigue. The “Brief Fatigue Inventory” (BFI) and the fatigue extension of the FACT-F (Functional Assessment of Cancer Therapy: Fatigue) are used in scientific research.
Short Form-36 Health Survey (SF-36)
The SF-36-Item Health Survey looks at eight concepts: physical functioning, role limitations due to physical health, role limitations due to personal or emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and overall health perceptions. A single item is included to gauge the perceived change in health. The answers to the questions have a weight tied to them with one getting a score of 0 and 5 a score of 100 or vice versa so that an answer with the score of 1 yields a weight of 100. Similar items get grouped together in the aforementioned categories and are averaged to form a scale. This is a good system for research but is not a quick way to assess the patient’s condition in the clinical setting (Ware 2000).
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30 (EORTC QLQ-C30)
The QLQ-C30 is a 30-item self-assessment questionnaire about the quality of life. Items include questions like “During the past week: Were you short of breath? Have you had pain? Did you need to rest?” with 28 of these questions scored from 1 (not at all) to 4 (very much). The last two items are scored from 1 to 7. Similar to the SF-36, some items have to be reversed to get all scales in the same direction and then computed in groups and single items: physical functioning, role functioning, social functioning, emotional functioning, cognitive functioning, fatigue, nausea and vomiting, dyspnea, sleeping disturbances, appetite loss, constipation, and diarrhea. This scoring system, again like with the SF-36, makes it difficult for physicians to draw conclusions at a glance (Jocham et al. 2009; “Questionnaires | EORTC” 2017; Aaronson et al. 1993).
Functional Assessment of Cancer Therapy: Fatigue (FACT-F)
The FACT-F is a 41-item questionnaire used to assess fatigue and anemia-related problems in patients suffering from cancer. It consists of the four groups of items from the general FACT: physical well-being (7 items), social/family wellbeing (7 items), emotional well-being (6 items), functional well-being (7 items), and the additional module on fatigue (13 items). Items, for example, “I feel weak all over.. .”, are scored on a 5-point Likert rating scale ranging from 0 (not at all) to 4 (very much). As with the questionnaires above, some items must be reversed for the scoring. As an example, the fatigue subscale is scored by summing the scores of the relevant items, multiplying by 13, and dividing by the number of items answered for the subscale score, with a higher score indicating a better quality of life. The questionnaire only requires a sixth-grade level of reading comprehension to complete and usually takes no longer than 10 min to ?nish (Yellen et al. 1997).
Brief Fatigue Inventory (BFI)
An explicit look will be taken at the BFI Score. The BFI was specifically developed for the evaluation of fatigue in patients with oncological illnesses and can be completed (by a patient with no cognitive disorders) in about 5 min. The questionnaire entails ten items and allows to capture six different dimensions that are affected by fatigue with the question “during the past 24 h, fatigue has interfered with your: general activity, mood, walking mobility, normal work (includes both work outside the home and daily chores), relations with other people, and enjoyment of life.” Each category is scored from 0 (does not interfere) to 10 (completely interferes), plus the three other questions relating to fatigue levels now, at worst time, and usually are also scored from 0 to 10 which leads to a maximum score of 90. Values between 30 and 40 indicate a moderate fatigue, while scores over 70 are typical for a severe fatigue. The advantage of the one-page BFI is that it is shorter than other questionnaires, lowering the burden for patients to complete it (Mendoza et al. 1999).
A quick screening can be performed on admission to judge a patient’s level of fatigue if they are presenting symptoms. For the screening, a short survey composed of two questions inquiring about the patient’s weakness (physical dimension) and tiredness (cognitive dimension) can be very effective and useful. If the screening is positive, a comprehensive assessment should be performed. Symptom severity should be evaluated, for example, with the BFI, to define the starting point before initiating treatment. The next step would be to identify treatable causes for fatigue and weakness. Figure 2 shows a suggested procedure for a fatigue screening, while Fig. 3 presents important aspects which require special attention during the taking of a history. Some laboratory parameters which could help to shed light onto treatable causes for fatigue are listed in Fig. 4.
The assessment should be repeated during causal and symptomatic treatment, with assessment intervals individually adjusted for the patient. The fatigue intensity will most likely change over the course of the disease trajectory, and periods of increased burden on the patient should be identified and the symptom management intensified accordingly. Reassessment also helps medical staff to evaluate the effectiveness of the treatment and make decisions regarding whether to initiate additional or alternative interventions if the current regimen shows inadequate effectiveness. As an objective assessment of fatigue is not possible, the patient is the only person who can assess their well-being. Subjective selfassessments of fatigue are an important part of the patient-reported outcome measures (PROMs) process, which is the gold standard of outcome measurement in palliative care (Bausewein et al. 2016). Unfortunately, in advanced stages of the disease, the patient’s cognitive abilities may be severely impaired, preventing the use of self-assessment questionnaires. In this setting, assessment may be done by family caregivers or medical staff.
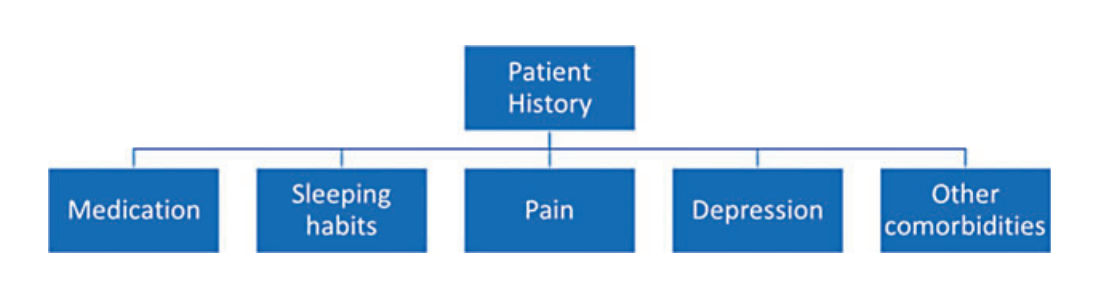
Fig. 3. Items which require special attention during a patient’s history
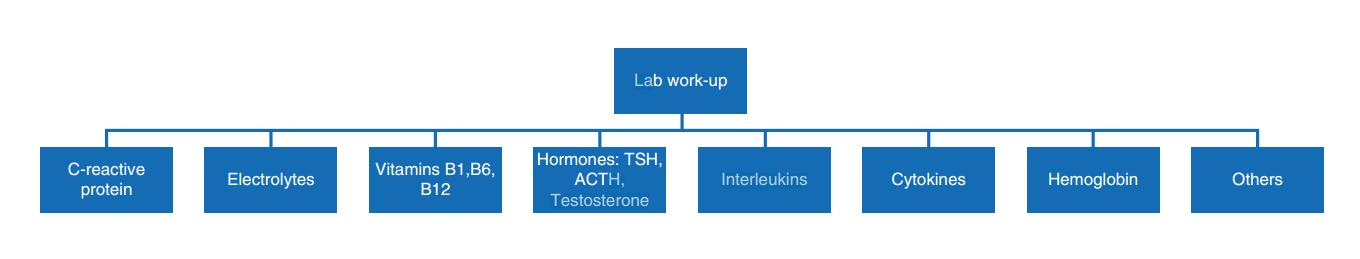
Fig. 4. Laboratory parameters suggested for routine assessment of fatigue
Therapy and management
Fatigue management is based on comprehensive internal medicine and oncological care, which includes the treatment of comorbidities, infections, hormonal and endocrine irregularities, and a review of the medication regimen. The underlying philosophy of palliative care should be considered in all aspects of assessment and treatment of fatigue. The patient’s individual needs and preferences should also be considered, and shared decision making should prevail in all situations. Discussions with the patient should take place along every step of the way, with them participating in all health care decisions. The physician should be able to clearly communicate the pros and cons of all available treatment options to the patient using language that the patient can understand so that informed consent and shared decision making can take place for all pharmaceutical as well as non-pharmaceutical interventions. The management of fatigue requires a high level of cooperation and communication between the health care provider, the patient’s family and friends, and the patient themselves. Therapeutic goals, for either pharmacological or non-pharmacological interventions, can only be achieved when all persons around the patient actively work together.
Fatigue in end-stage terminally ill patients should be viewed as a natural part of the dying phase and not be considered a symptom that requires treatment. In this setting, fatigue may ease the transition to death, shielding the patient from distress, anxiety, and burdening symptoms. Symptomatic treatment of fatigue in dying patients could worsen the patient’s well-being by forcing them to experience the last excruciating hours of their lives in full detail. Medical staff should recognize the appropriate time to stop treatment for fatigue to allow patients to die without distress (Mьcke et al. 2016).
General supportive measures when treating fatigue
A fatigue diary is a useful tool in the management of fatigue. This diary should include a timeline for each day, the activity that was being performed and at what time it was performed, a comments section, and separate columns for intensity of weakness and tiredness. These last two columns should use simple scales such as a four-step categorical scale (no = 0, slight = 1, moderate = 2, severe = 3, intensity of weakness/tiredness). The diary serves as a visualization of the symptom burden and allows patients to better plan their energy reserves when they become familiar where the main limitations are. For the health care team, it allows the monitoring of the effectiveness of interventions.

Fig. 5. (a) Shows unappealing, sloppily prepared food that would most likely dampen the appetite of a healthy individual. (b) Presents an esthetically pleasing meal which does not take much more effort to prepare than A but is more likely to be appetizing
A sufficient intake of water and vitamin supplements has been discussed as having a positive effect on fatigue, although there are currently no scientific studies to support this (Mochamat et al. n.d.). Drinking more fluids has been hypothesized to increase the elimination of fatigue inducing metabolites which form during chemoand radiotherapy or other processes. Most palliative care patients suffer from a vitamin deficiency, so that a substitution with multivitamins and minerals can be justified in most cases. Nutrition plays another key role in the energy imbalance of fatigue. Diverse and varied meals should be prepared and administered in small portions. Sometimes the support of nutritional specialists and ecotrophologists (specialists in nutrition, household management, and home economics) may be needed. Clinical experience has shown that the way food is presented plays an important role on appetite in severely ill patients with appetite disorders. This is demonstrated in Fig. 5, which meal would you rather eat? Patients with severe fatigue prefer meals that do not require much effort to chew and are easy to swallow. Studies have shown that appetite and cachexia may influence each other and that treatment of appetite loss with megestrol acetate can decrease fatigue intensity. Beverages such as coffee, tea, or soft drinks can have a stimulating and pleasing effect on the patient. Alcohol, especially in the form of a predinner apéritif, has the potential to kick-start the appetite so that a glass of wine or beer is potentially recommended for those patients who report this as favorable from their history. Air quality, temperature, smells, sounds, light, and colors can also have profound effects on the well-being and should be utilized in the management of fatigue.
Recommendations for pharmacological therapies
Treatable causes of fatigue should be excluded before symptomatic pharmacological treatment of fatigue is initiated (Fig. 1). Anemia should be treated with iron supplements, vitamin B12 substitution, or folic acid depending on the cause. Even blood transfusions or erythropoietin substitution (EPO) can be considered albeit with rigid indications. Electrolyte imbalances should be corrected, and infections should be adequately treated. Endocrine disorders such as adrenocortical in sufficiency or a hypothyroidism should be substituted with the respective treatment. Side effects of medications such as analgesics (especially opioids), sedatives, anticonvulsants, antiemetics, or antihypertensive drugs should be kept in mind as causes or enhancers of fatigue, and switching to similar medications with fewer or less severe side effects should be considered.
Symptomatic pharmacological treatment of fatigue is restricted to very few options. Two Cochrane reviews have summed up the evidence on this topic (Mьcke et al. 2016; Minton et al. 2010), but were not able to present clear recommendations. There are a sufficient number of studies included in these reviews, but patient numbers were small or patients from heterogenous groups were included. The comparability of the included studies was difficult due to a wide range of assessment instruments used.
Methylphenidate was evaluated in five studies including cancer patients (Bruera et al. 2006; Butler et al. 2007). One study showed no significant effect with a daily dose of 18–54 mg/day, while the other four displayed a slight improvement under a continuous intake of dosages between 5 and 30 mg/day. The mechanism of action of methylphenidate includes the inhibition of noradrenaline and dopamine reuptake in the synaptic cleft, thus resulting in increased sympathetic nervous system activity. But noradrenaline and dopamine seem not to be the only systems affected by methylphenidate; research suggests it plays another role as a serotonin receptor agonist.
Modafinil seemed to be able to effectively treat severe fatigue with dosages of 100 and 200 mg/ day according to two studies (Lange et al. 2009; Spathis et al. 2014), but had no effect on mild to moderate fatigue. Similarly, modafinil was not effective in treating CFS patients with lung cancer. The therapeutic effect of modafinil is attributed to its ability to affect the sleep-wake rhythm, but due to its numerous side effects, which include severe psychiatric symptoms and cutaneous reactions, its indication is limited to the treatment of profound drowsiness in adults.
Taking a look at corticosteroids for the treatment of fatigue, dexamethasone and methylprednisolone were only included in two studies although these two drugs are recommended in official guidelines and extensively used in clinical practice. It is assumed that glucocorticoids play a positive role in managing tumor-associated fatigue due to their ability to influence the central nervous system and their anti-inflammatory attributes (Shih and Jackson 2007). A recent study demonstrated that 4 mg/day of dexamethasone was significantly superior to placebo (Yennurajalingam et al. 2013). However, there was no significant difference in the improvement of individual symptoms, psychological distress, anxiety, and depression in the dexamethasone group compared with placebo. Further studies examining the effects of dexamethasone on fatigue are needed.
Mistletoe extract PS76A2 improved fatigue symptoms in a study including breast cancer patients (Marvibaigi et al. 2014), while thyreoliberin (thyrotropin-releasing hormone, TRH) was also successfully tested but has yet to receive licensing. TRH exhibited an important influence on the central regulation of energy homeostasis, inflammatory processes of the organism, and fatigue. This is most likely due to its extra-hypothalamic and central nervous system activity (Kamath et al. 2009). Ginseng showed positive results in a few studies. Its possible pharmacological effects in the treatment of CFS are theorized to lie in its affinity to glucocorticoid receptors, its membrane stabilizing effect, and its anti-inflammatory feature (Radad et al. 2011; Rasheed et al. 2008). Erythropoietin was, until 2004, globally one of the most successful drugs for cancer-related fatigue. Unfortunately, in 2009 a meta-analysis consisting of 53 clinical trials with almost 14,000 patients concluded that the mortality rate of cancer patients after receiving EPO treatment increased by a factor of 1.17. This may be related to EPO receptors that were found on various tumor cell surfaces, leading to the hypothesis that EPO could stimulate malignant neoplasm growth. Not only did EPO increase the likelihood of tumor progression, but it also contributed to a higher risk of venous thromboembolisms in patients with solid mass tumors. This restricted the use of EPO to symptomatic adult patients with therapy-induced anemia, but only for hemoglobin levels below 12 g/dl.
Acetylsalicylic acid (aspirin, ASA) showed impressive results in the treatment of fatigue in patients with multiple sclerosis (Wingerchuk et al. 2005; Shaygannejad et al. 2012), but, according to the latest publications, has not yet been tested for CRF. Paroxetine was investigated in one study with cancer patients (Morrow et al. 2003) and another with patients suffering from chronic obstructive pulmonary disease (COPD), but neither study was able to confirm a significant improvement. In consequence, selective serotonin reuptake inhibitor (SSRI) antidepressants only seem to present a therapeutic option when a depressive disorder is diagnosed. The systematic review also found no significant improvements for drugs like testosterone, amantadine, carnitine, megestrol, or medroxyprogesterone.
Thalidomide has become a talking point as a treatment for cachexia in tumor patients but has so far failed to show tangible results (Davis et al. 2012; Reid et al. 2012; Yennurajalingam et al. 2012).
Based on limited evidence, we cannot recommend a specific pharmaceutical for the treatment of fatigue in palliative care patients. Fatigue research in palliative care seems to focus on modafinil and methylphenidate, which may be beneficial, although further research regarding their efficacy is needed. Dexamethasone, methylprednisolone, acetylsalicylic acid, armodafinil, amantadine, and L-carnitine should also be further examined.
Non-pharmacological management
The preservation and protection of available energy reserves as well as the patient’s selfreliance in day-to-day activities plays an especially important role for tumor patients with fatigue. The proper management includes a conscious intake of calories and a protein-rich diet, as well as maintenance of a proper sleep routine which avoids caffeinated drinks before bedtime, too much daytime sleep, or arousing activities in the late afternoon or evening. The patient will need time to get used to this new activity and energy management. Priorities must be set to differentiate between useful/important and less important activities. Energy and stamina should be rationed accordingly and necessary breaks between activities planned for. Moderate physical exercise is useful, even for cancer patients suffering from exhaustion and reduced physical ability, in order to retain existing physical abilities and to counteract progressive muscle loss, reduced efficiency of the cardiovascular system, and general condition. Multiple studies have shown the benefits of light endurance training during anticancer therapy (Hil?ker et al. 2017; Safari et al. 2017) regardless of the type of exercise. Physiotherapy can contribute significantly to the patient’s wellbeing with mobilization, breathing exercises, and teaching techniques for self-training. On the other hand, the use of medical aids such as a wheelchair or walker has to be learned and accepted. Psychosocial measures such as stress management and relaxation therapy (autogenic training, progressive muscle relaxation, or yoga) contribute to the management of CRF. Cognitive behavioral therapies are also of great value. Psychosocial support from caregivers is, likewise, beneficial to relieve the patient from fears and concerns. However, a recent review from 2017 was unable to recommend any specific non-pharmacological intervention, although physical exercise and psychoeducational therapies seem to be promising options and should be researched further.
Conclusion
Fatigue is a subjective feeling of extraordinary weakness, tiredness, and energy loss which affects the body (physical aspect), feelings (affective aspect), and mental function (cognitive aspect) and can occur at any age. Fatigue is not, or only incompletely, alleviated by sleep and rest. CRF is a subjective, multidimensional illness, with significant impact on the quality of life of patients. Once treatable causes for CRF are excluded, a multimodal treatment regimen should be developed. Symptomatic management of fatigue requires a high level of cooperation and communication between all health care professionals attending to the patient, the patient’s social sphere, and the patient themselves. Treatment strategies must be multimodal and multi-professional. Past systematic reviews have provided no clear recommendations for pharmacological or non-pharmacological interventions for the relief of cancer-related fatigue. Treatment of underlying causes, such as infections, may be an effective measure to reduce fatigue symptoms. Current ongoing studies are exploring therapeutic options such as energy homeostasis, glucocorticoid receptor stimulation, as well as influencing neurotransmitter receptors and anti-inflammatory processes. Physical training can be effective and should be used in combination with pharmaceutical interventions.
References
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85 (5):365–76.
Bausewein C, Daveson BA, Currow DC, Downing J, Deliens L, Radbruch L, De?lippi K, et al. EAPC white paper on outcome measurement in palliative care: improving practice, attaining outcomes and delivering quality services – recommendations from the European Association for Palliative Care (EAPC) task force on outcome measurement. Palliat Med. 2016;30 (1):6–22. https://doi.org/10.1177/0269216315589898.
Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, et al. Cancerrelated fatigue, version 2.2015. J Natl Compr Cancer Netw. 2015;13(8):1012–39.
Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(0): S48–57. https://doi.org/10.1016/j.bbi.2012.06.011.
Bruera E, Valero V, Driver L, Shen L, Willey J, Zhang T, Lynn Palmer J. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebocontrolled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(13):2073–8. https://doi.org/10.1200/ JCO.2005.02.8506.
Butler JM, Douglas Case L, Atkins J, Frizzell B, Sanders G, Griffin P, Lesser G, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of D-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1496–501. https:// doi.org/10.1016/j.ijrobp.2007.05.076.
Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park). 1998;12(11A): 369–77.
Cella D, Davis K, Breitbart W, Curt G, Coalition F. Cancerrelated fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19 (14):3385–91. https://doi.org/10.1200/ JCO.2001.19.14.3385.
Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early ?ndings. Brain Behav Immun. 2008;22(8):1197–200. https://doi.org/ 10.1016/j.bbi.2008.05.009.
Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, and Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. The national academies collection: reports Funded by National Institutes of Health. Washington, DC: National Academies Press (US); 2015. http://www.ncbi.nlm.nih.gov/books/ NBK274235/.
Davis M, Lasheen W, Walsh D, Mahmoud F, Bicanovsky L, Lagman R. A phase II dose titration study of thalidomide for cancer-associated anorexia. J Pain Symptom Manag. 2012;43(1):78–86. https:// doi.org/10.1016/j.jpainsymman.2011.03.007.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. https://doi.org/ 10.1016/S1470-2045(10)70218-7.
Glaus A. Fatigue in patients with cancer. Vol. 145. Recent Results in Cancer Research. Berlin: Springer; 1998. https://doi.org/10.1007/978-3-642-51466-1.
Hil?ker R, Meichtry A, Eicher M, Nilsson BL, Knols RH, Verra ML, Taeymans J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirectcomparisons meta-analysis. Br J Sports Med. 2017. https://doi.org/10.1136/bjsports-2016-096422.
Jocham HR, Dassen T, Widdershoven G, Halfens R. Reliability and validity of the EORTC QLQ-C30 in palliative care cancer patients. Cent Eur J Med. 2009;4 (3):348–57. https://doi.org/10.2478/s11536-009-00327.
Kamath J, Yarbrough GG, Prange AJ, Winokur A. Thyrotropin-releasing hormone can relieve cancerrelated fatigue: hypothesis and preliminary observations. J Int Med Res. 2009;37(4):1152–7. https://doi. org/10.1177/147323000903700420.
Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009;256(4):645–50. https://doi.org/10.1007/ s00415-009-0152-7.
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. JNCI Monogr. 2004;2004(32):40–50. https://doi.org/10.1093/ jncimonographs/lgh027.
Marvibaigi M, Supriyanto E, Amini N, Majid FAA, Jaganathan SK. Preclinical and clinical effects of mistletoe against breast cancer. Bio Med Res Int. 2014. https://doi.org/10.1155/2014/785479.
Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. 1999;85(5):1186–96.
Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. In: Cochrane database of systematic reviews: Wiley; 2010. https://doi.org/10.1002/14651858. CD006704.pub3.
Mochamat MM, Cuhls H, Conrad R, Kravchenko D, Radbruch L. Non-pharmacological treatments for fatigue in advanced disease associated with palliative care: a systematic review. (Unpublished data).
Mochamat, Cuhls H, Marinova M, Kaasa S, Stieber C, Conrad R, Radbruch L, Mьcke M. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: a European Palliative Care Research Centre cachexia project. J Cachexia Sarcopenia Muscle. 2017;8 (1):25–39. https://doi.org/10.1002/jcsm.12127. Epub 2016 Jul 20. Review. PubMed PMID: 27897391; PubMed Central PMCID: PMC5326814.
Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PLR, Flynn PJ, Hynes HE, Banerjee TK, Kirshner JJ, King DK. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21(24):4635–41. https://doi.org/10.1200/ JCO.2003.04.070.
Mьcke M, Mochamat HC, Peuckmann-Post V, Minton O, Stone P, Radbruch L. Pharmacological treatments for fatigue associated with palliative care: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2016;7(1):23–7. https:// doi.org/10.1002/jcsm.12101.
Questionnaires | EORTC. 2017. http://groups.eortc.be/qol/ eortc-qlq-c30. Accessed 25 May.
Radad K, Moldzio R, Rausch W-D. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17(6):761–8. https://doi.org/10.1111/j.1755-5949.2010.00208.x.
Radbruch L, Strasser F, Elsner F, Gonзalves JF, Lшge J, Kaasa S, Nauck F, Stone P. Fatigue in palliative care patients – an EAPC approach. Palliat Med. 2008;22 (1):13–32. https://doi.org/10.1177/ 0269216307085183.
Rasheed N, Tyagi E, Ahmad A, Siripurapu KB, Lahiri S, Shukla R, Palit G. Involvement of monoamines and proinflammatory cytokines in mediating the anti-stress effects of panax quinquefolium. J Ethnopharmacol. 2008;117(2):257–62. https://doi.org/10.1016/j. jep.2008.01.035.
Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, Donnelly M. Thalidomide for managing cancer cachexia. In: Cochrane database of systematic reviews: Wiley; 2012. https://doi.org/10.1002/14651858. CD008664.pub2.
Safari R, Van der Linden ML, Mercer TH. Effect of exercise interventions on perceived fatigue in people with multiple sclerosis: synthesis of meta-analytic reviews. Neurodegener Dis Manag. 2017;7(3):219–30. https:// doi.org/10.2217/nmt-2017-0009.
Shaygannejad V, Janghorbani M, Ashtari F, Zakeri H. Comparison of the effect of aspirin and amantadine for the treatment of fatigue in multiple sclerosis: a randomized, blinded, crossover study. Neurol Res. 2012;34(9):854–8. https://doi.org/10.1179/1743132812Y.0000000081.
Shih A, Jackson KC. Role of corticosteroids in palliative care. J Pain Palliat Care Pharmacother. 2007;21 (4):69–76.
Spathis A, Fife K, Blackhall F, Dutton S, Bahadori R, Wharton R, O’Brien M, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebocontrolled, double-blind, randomized trial. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32 (18):1882–8. https://doi.org/10.1200/ JCO.2013.54.4346.
Stark L, Tofthagen C, Visovsky C, McMillan SC. The symptom experience of patients with cancer. J Hosp Palliat Nurs. 2012;14(1):61–70. https://doi.org/ 10.1097/NJH.0b013e318236de5c.
Stiel S, Matthes ME, Bertram L, Ostgathe C, Elsner F, Radbruch L. Validierung der neuen Fassung des Minimalen Dokumentationssystems (MIDOS2) fьr Patienten in der Palliativmedizin. Der Schmerz. 2010;24(6):596–604. https://doi.org/10.1007/s00482-010-0972-5.
Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer Palliat Med Art Sci. 2008;44(8):1097–104. https://doi. org/10.1016/j.ejca.2008.02.037.
Wang XS. Pathophysiology of cancer-related fatigue. Clin J Oncol Nurs. 2008;12(5 Suppl):11–20. https://doi.org/ 10.1188/08.CJON.S2.11-20.
Ware JE. SF-36 health survey update. LWW Spine. 2000;25(24):3130–9.
Wingerchuk DM, Benarroch EE, O’Brien PC, Keegan BM, Lucchinetti CF, Noseworthy JH, Weinshenker BG, Rodriguez M. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267–9. https://doi.org/10.1212/01. WNL.0000156803.23698.9A.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13(2):63–74. https://doi.org/10.1016/ S0885-3924(96)00274-6.
Yennurajalingam S, Willey JS, Lynn Palmer J, Allo J, Del Fabbro E, Cohen EN, Tin S, Reuben JM, Bruera E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study. J Palliat Med. 2012;15(10):1059–64. https://doi.org/ 10.1089/jpm.2012.0146.
Yennurajalingam S, Susan F-H, Lynn Palmer J, DelgadoGuay MO, Bull J, Phan AT, Tannir NM, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31 (25):3076–82. https://doi.org/10.1200/ JCO.2012.44.4661.