Введение
Humans are social animals and nearly all our social situations revolve around conversation and food. This presents a real problem when you have swallowing difficulties when it can be embarrassing, unsafe, or anxiety-provoking to consider a meal with friends and family. When patients face limited time, these situations become even more acute, and quality of life (particularly with eating and drinking) is of paramount consideration.
Problems with swallowing can develop for many reasons in patients nearing the end of life. These include the direct effect of the illness, effects of treatment or management, cognitive issues, medication effects, deconditioning and fatigue, change in taste, loss of saliva, mood disorders, and inanition. The clinician should be particularly cognizant of deglutitive changes and inquire about these directly as they often impact quality of life and, in many cases, there are options to ameliorate symptoms. Managing swallowing problems is a multidisciplinary challenge. Teamwork can be the critical factor in preventing lifethreatening complications and long-term morbidities and in offering support and timely intervention. Regular and clear communication between team members and the patient can relieve anxiety, provide education, and optimize function.
In this chapter, we will review the importance and impact of deglutition and swallowing dysfunction in the environment of palliative care. This will provide a practical platform for clinicians who care for those receiving palliative care which includes anticipating possible problems, recognizing symptoms and signs of swallowing disorders, and identifying management strategies and options.
Глотание
Swallowing is a complex process requiring a series of muscles to work in synchronicity to perform a relatively short movement. Some movements are voluntary, and others reflexive, but they are codependent (Logemann 1998).
Нормальное глотание
It should first be noted that the “normal” swallow shows great variability among healthy individuals. Deglutition involves 30 muscle pairs, 5 cranial nerves and accessory nerves, coordinating with cortical, subcortical, and medullary respiratory centers and the swallow central pattern generator (Shaw and Martino 2013). The complexity of deglutition and its need to coordinate precisely with respiration results in a series of protective airway reflexes and responses. Disordered deglutition is an airway risk and may result in significant morbidity and mortality. Understanding neurologic control points enables better management of patients with swallowing problems. A summary of cranial nerve function involved with swallowing is provided below. Deglutition has been arbitrarily divided into phases. However, in reality, it is a continuous process, and many aspects occur simultaneously within a predetermined sequence. Recent research indicates the interdependent aspects of deglutition should be treated as a continuum, rather than in separate phases. Nonetheless, historically swallowing has been divided into four phases (oral preparatory, oral, pharyngeal, and esophageal).
Assessment of all aspects is required to understand swallowing ability and dietary/nutrition risk.
The swallow begins with a person being alert and able to sense food. It is common practice to include both the oral preparatory phase and oral phase under a single heading in an assessment, namely, the oral phase. This is considered the voluntary stage/s of the swallow where bolus may be voluntarily ejected from the oral cavity.
Food is placed in the mouth and the lips are closed to prevent anterior spillage. The food or fluid in the mouth is then masticated (chewed) and mixed with saliva to create a cohesive bolus. The bolus is held between the tongue and the anterior hard palate. Mastication is in a lateral rotary movement, and the tongue manipulates the bolus to the back of the mouth. The bolus is held in place by contraction of the soft palate against the base of the tongue and contraction of the buccal muscles. Dentition influences deglutition by contribution to bolus breakdown, allowing salivary enzymes to begin digestion, tastants to be sensed, airway closure to begin, and lubrication to be provided to form a cohesive bolus.
The tongue begins to move the bolus toward the back of the mouth, against the hard palate. Negative pressure is created by muscle contraction, and the bolus moves toward the faucial pillars.
Innervation is predominantly via the trigeminal nerve (CNV), the facial nerve (CNVII), and the hypoglossal nerve (CNXII). The vagus nerve (CNX) innervates the palatoglossus muscle. CNVII innervates the muscles of the face, while CNV innervates those muscles used to chew, elevate, and lateralize the mandible. The intrinsic and extrinsic muscles of the tongue are innervated by CNXII (other than the palatoglossus). Finally, the soft palate is innervated by CNVand CNX (Drake et al. 2010; Moore 2010). Sensation is provided by the lingual nerve (a branch of CNV) and the glossopharyngeal nerve (CNIX) with special taste senses provided by the chorda tympani (branch of CNVII, hitchhiking with the lingual nerve) and CNIX. Sensation of the valleculae is via CNX, superior laryngeal nerve. This nerve is critical in guarding the larynx and mediating laryngeal closure and airway protection when bolus or secretions are identified.
The swallowing reflex is initiated as the bolus passes the faucial arches triggering the pharyngeal phase of the swallow, which is stereotyped, involuntary, and centrally coordinated. Total pharyngeal transit time in adults is usually less than 1.5 s and must encompass airway closure, laryngeal elevation, pharyngeal peristalsis, and pharyngoesophageal segment (PES) opening. This requires coordination with the respiratory system.
The soft palate retracts to close the velopharyngeal port. The bolus passes over the base of the tongue, which retracts toward the posterior pharyngeal wall. The pharyngeal muscles create peristaltic waves traveling at approximately 15 m/s, which carry the bolus through the pharynx. Simultaneously, the epiglottis Deflects the bolus laterally to protect the laryngeal vestibule and retroverts which smoothes the valleculae. The aryepiglottic folds, false and true vocal folds all contract to achieve airway closure and prevent penetration and aspiration. The hyoid moves anterosuperiorly (approximately the distance of one vertebra) which acts as a drawstring to assist in distension of the upper esophageal sphincter (UES). Cessation of tonic UES contraction occurs within 0.3 s after suprahyoid muscular contraction starts to ensure an open sphincter when the bolus arrives. Bolus size influences duration of hyolaryngeal elevation and airway closure duration, with larger volumes resulting in greater opening duration and displacement.
The vagus nerve transmits pharyngeal sensory information via pharyngeal and superior laryngeal branches and is the key mediator of the airway closure reflex (adductor reflex). Afferent information converges at the nucleus tractus solitarius (NTS) of the medulla and then travels through interneurons to the nucleus ambiguous (NA) which produces efferent (motor) output. Motor signals return to the pharynx via vagal branches (pharyngeal plexus and recurrent laryngeal nerve), with additional input from the dorsal motor nucleus (DMN) of the vagus (inhibitory). The DMN input likely prevents mis-sequencing. Vagal outflow also initiates airway protective mechanisms, and accessory nerve and C1 and C2 nerves produce hyoid elevation (Drake et al. 2010; Moore 2010).
Relaxation of the upper esophageal sphincter occurs and lasts less than 1 s. In reality this is a cessation of tonic contraction, rather than an active relaxation (Allen 2016; Miles et al. 2016). The UES then contracts to protect the pharynx from regurgitated material and to limit air swallowing. The bolus is carried via esophageal peristalsis (circular and longitudinal muscle contractions) to the lower esophageal sphincter (LES) (Shaw and Martino 2013). Once the LES has relaxed, the bolus moves into the stomach. The process of transportation through the esophagus takes from 8 to 20 s for liquid but may be as long as 60 s for pills or solids (Logemann 1998; Miles et al. 2015, 2016).
CNX mediates UES function allowing bolus into the esophagus during deglutition (Drake et al. 2010; Moore 2010). Esophageal peristalsis is under vagal and myenteric control through the NA and DMN of the vagus combined with sympathetic and parasympathetic neural networks. Differential latencies and a pool of inhibitory vagal neurons enable a sequential waveform to occur from proximal to distal within the esophagus.
The disordered swallow
The “normal” swallow demonstrates variability which, at times, can be difficult to differentiate from disordered deglutition. Physical trauma, tumors, neuromuscular conditions, surgery, medicines, and aging all contribute to changes in a swallow.
Типичные черты нарушения глотания
Change to the musculature or innervation of the swallow may result in disordered deglutition. Ultimately, the purpose of a swallow is to pass food and fluids from the mouth to the stomach via the pharynx and esophagus. Given the interdependence of the swallow phases, it follows that disruption to any stage may result in dysphagia (swallowing problems) (Таблица 1).
Altered oral sensation may yield anterior spillage of a bolus, incomplete mastication, oral incompetence, and delayed initiation of a swallow (if a swallow is initiated at all). Problems with lateralization of the jaw, or rotary mastication, or damaged dentition may give rise to an incompletely masticated or two-phase/piecemeal bolus, which poses a risk of aspiration. A cohesive bolus is required for safe transportation throughout a swallow, as a piecemeal bolus heightens the risk of pooling, residue, and material breaking away into the laryngeal vestibule. A two-phase bolus may compromise airway closure mechanisms due to differential bolus velocities and therefore confusion over appropriate airway closure duration.
Saliva is critical in mastication, helping to achieve a cohesive bolus, providing lubrication, initiating digestion, enabling tastant dissolution, mechanically flushing the oral cavity, and protecting dentition and mucosal lining. One need only see a patient following radiotherapy with xerostomia and its significant impact on oral bolus control and dental health to appreciate the benefit of saliva.
If oral incompetence is present, bolus transit may be affected, either with anterior loss (i.e., from the lips) causing skin irritation and embarrassment or inappropriate posterior spillage into the pharynx with potential for airway soiling. Velopharyngeal incompetence will also affect oral transit, as the bolus may escape North into the nasopharynx. Loss of the sтаблица velopharyngeal diaphragm impairs pressure generation and ability to provide impetus to the bolus in the oropharynx. Residue may be observed in the valleculae or tongue-base regions or pyriform apices and intra-deglutitive or post-deglutitive aspiration of residue may occur.
From the time that bolus contacts the tongue dorsum, airway closure begins. Once the bolus reaches the posterior third of the tongue, the involuntary swallow is triggered. A coordinated sequence of events occurs. This begins with three-level airway closure, hyolaryngeal elevation and consequent epiglottic retroversion, and pharyngeal constrictor peristalsis. Cessation of upper esophageal sphincter contraction enables distraction of the UES and generation of negative pressure in the pharyngoesophageal segment which combine to effect bolus transit. This all occurs in approximately 1.5 s, and even slight mismatching of event timing may have significant ramifications. Consequences include misdirection of bolus ю/— airway violation, incomplete bolus transit with residue which may then be aspirated, and anatomic changes such as development of a diverticulum. Poor sensation due to neurological disease, surgical distortion, and post-radiotherapy damage or injury will prevent the SLN from identifying incoming bolus in a timely fashion. Airway closure may then be late or absent and aspiration may occur. Loss of strength in pharyngeal musculature reduces impetus of the bolus and means it must rely on gravity and tongue pumping to coincide with UES opening and allow bolus flow. Anterosuperior hyoid elevation and cessation of tonic UES contraction result in a distracting effect, opening the UES. If hyolaryngeal elevation is present, this may be enough to permit bolus transit, but if there is poor elevation or mistiming of bolus arrival at the UES, then penetration and aspiration will ensue. At times only part of the bolus may traverse the UES leaving bolus remnants in the pyriform fossae. Given that the larynx descends post-deglutition and protective airway constriction relaxes, this can expose the airway to misdirected bolus. Surgical tethering of tissue and radiation fibrosis often limit hyolaryngeal elevation. Stroke or progressive neurologic disease frequently impairs pharyngeal strength and results in reduced UES opening duration and diameter.
Таблица 1. Распространенные причины нарушения глотания
| Contributor | Effect | Complaint |
| Mass or tumor |
Deflect bolus incorrectly
Obstruct deglutitive pathway Neural compromise – sensory and motor Pain – reduced effort and mobility of pharynx Loss of airway protection |
Solid and liquid dysphagia
Pill dysphagia Lump in the throat Cough Loss of appetite Choking |
| Dryness (xerostomia, xerophagia) |
Mucosal irritation (abrasion)
Thick mucus Reflux Impaired bolus cohesion and transit |
Pain
Cough and hawking Acid or bile taste, burning pharynx/chest pain Food sticking or spitting out food |
| Medications |
Dryness
Drowsiness Loss of appetite Constipation |
Solid food dysphagia
Loss of appetite Weight loss Early satiety |
| Cognitive issues |
Reduced urge to eat
Depression – loss of appetite Fear of choking |
Weight loss Low mood
No interest in food |
| Deconditioning |
Loss of muscle tone and strength
Loss of compensatory reserve |
Solid and liquid dysphagia
Weight loss Fatigue Reduced volume of food intake |
If material enters the laryngeal vestibule, then penetration has occurred. When material passes the vocal folds into the airway, aspiration has occurred. Either incident may be associated with a response to eject the material, and in many cases bolus penetration is simply cleared during the swallow by the closure of the airway “squeezing” bolus out of the larynx. If there is no response, then aspiration is deemed “silent.” This presents the greatest risk as bolus may remain lodged in the lower airways and produce pneumonitis, lung abscess, pneumonia, or death.
UES constriction persists after bolus passage to ensure no retrograde movement of the bolus into the pharynx (esophagopharyngeal reflux, EPR). Poor peristaltic wave in the esophagus may impair bolus transport and risk reflux of the material into the pharynx. This may prompt compensatory UES hyperfunction as a protective mechanism to prevent pharyngeal transgression. In some patients this is felt and described as a lump on the throat or globus sensation. If mechanical obstruction is present in the esophagus (e.g., unfolded or stiff aorta; peptic, malignant, or caustic stricture; Schatzki’s rings; or external compression), bolus may also be impeded at that site.
Assessment of esophageal function may be performed by various individuals and varies between centers. For example, in Australia, it is formally the domain of the gastroenterologist. However, in New Zealand, otolaryngologists, gastroenterologists, radiologists, and speech pathologists may assess and comment on the esophageal phase of swallowing.
Таблица 2. Шкала пенетрации-аспирации
| Шкала | Описание реакций |
| 1 | Material does not enter the airway |
| 2 | Material enters the airway, remains above the vocal folds, and is ejected from the airway |
| 3 | Material enters the airway, remains above the vocal folds, and is not ejected from the airway |
| 4 | Material enters the airway, contacts the vocal folds, and is ejected from the airway |
| 5 | Material enters the airway, contacts the vocal folds, and is not ejected from the airway |
| 6 | Material enters the airway, passes below the vocal folds, and is ejected into the larynx or out of the airway |
| 7 | Material enters the airway, passes below the vocal folds, and is not ejected from the trachea despite effort |
| 8. | Material enters the airway, passes below the vocal folds, and no effort is made to eject |
Аспирация и пенетрация
Aspiration and penetration describe entry of bolus material into the larynx. Penetration occurs when bolus crosses the plane of entry of the laryngeal vestibule (a line following the aryepiglottic folds to the epiglottic tip), and aspiration when the bolus passes beneath the vocal folds. A response may occur in either situation, e.g., a cough to clear; however, if a response is lacking, then silent aspiration has occurred. This is a major risk factor for pulmonary problems as bolus remains within the lower airways. Rosenbek and colleagues described the penetration-aspiration scale (Rosenbek et al. 1996) which categorizes the degree of airway violation and whether a response is seen (Таблица 2). This is one of many forms of categorizing severity of penetration and aspiration.
A major goal of dysphagia management is to prevent adverse consequences of aspiration, namely, fever, decreased oxygen saturation, chest infections, aspiration pneumonia, decreased quality of life, increased length of stay, and death. It is also important to note the additional financial burden that dysphagia imparts on the healthcare system. Although no data is available for palliative care, international data estimates that the cost of dysphagia per person, within 1 year of ischemic stroke, is $4510 more than those without dysphagia (Bonilha et al. 2014). Additionally, Sutherland, Hamm, and Hatcher (2010) estimated the mean cost of treatment for a person with aspiration pneumonia to be $17,000. Although these figures do not dictate the management of a person with dysphagia, it is an important administrative fact in advocacy for early dysphagia management.
Дисфункция глотания при определенных заболеваниях
Although aging alters swallow physiology, many older adults maintain functional swallows well into their tenth decades. Certain high-risk illnesses are associated with increased swallowing problems, and management is determined by the underlying causes, patient factors, and resources. Some of these conditions are discussed in the following sections.
Рак головы и шеи
Representing around 5% of all cancers in Australasia, head and neck cancer is most commonly squamous cell carcinoma (HNSCC) arising from the epithelial lining of the upper aerodigestive tract. There is also a contribution from skinrelated carcinoma which may involve deeper structures and thyroid carcinoma which is intimately related to the recurrent laryngeal nerve putting the voice and swallow safety at risk. Risk factors for head and neck cancer include male gender, age >60 years, smoking, alcohol intake (and a combination of the two), and human papilloma virus (HPV) infection. Subsites within the head and neck vary as to which causative factor is most commonly responsible; e.g., oropharyngeal carcinoma is associated with HPV infection in >80% of cases now (Gillison et al. 2015). There is variation in treatments of sites, responses to treatment, and subsequent functional deficits that follow treatment. This is seen most starkly in the differential treatment response of HPV-positive carcinoma of the head and neck vs non-HPV positive carcinoma. In the last decade, HPV has been identified as a major contributor to disease in younger, nonsmoking, nondrinking individuals. The upside to this early onset disease is that it is typically far more responsive to treatment and demonstrates a greater cure rate. This has now led to trials in which therapy is being de-escalated to evaluate whether oncologic control can be achieved with a lower treatment burden. The aim is to reduce treatment-related morbidity such as dysphagia, voice change, osteoradionecrosis, and secondary malignancy (Mirghani et al. 2015; Masterson et al. 2014).
Malignancy at any subsite of the head and neck affects deglutition. Oral cavity lesions create pain on tongue motion; affect sensation, taste, and oral competence; and disturb mastication. Oropharyngeal malignancy may be obstructive and painful and reduce superior laryngeal nerve sensation leading to increased risk of aspiration. Hypopharyngeal tumors often result in obstruction of the pyriform apex or PES and may reduce vocal fold mobility compromising airway protection. Laryngeal cancer impairs airway protection reflexes and cough efficiency. Dysphonia is often an early sign in glottic-level tumors (Figs. 1 and 2) but may not be seen in supraglottic malignancy until advanced disease is present.
Most importantly in head and neck cancer patients, the treatment is also highly likely to impair both deglutition and phonation. Whether the treatment is surgical resection, radiation/ chemoradiation, or a combination of surgery and postoperative radiotherapy, vital structures will be in the “line of fire” at all times. Achieving cure of the cancer does not guarantee a functional swallow or voice. In many patients, swallowing parameters deteriorate in the 5 years following completion of treatment for HNSCC regardless of cancer eradication. Radiation-induced xerostomia and osteoradionecrosis are unique complications seen only following HNSCC treatment, not from the tumor itself. Xerostomia exposes dentition to increased risk of caries, periodontal disease, and tooth loss, further impairing oral bolus control and mastication. Radiation may also induce trismus such that mouth opening is grossly reduced and fitting food or a toothbrush into the oral cavity can be difficult. Resection of tongue or pharyngeal tissue may create sumps, insensate regions, and poor mobility even if reconstruction occurs.
Нервно-мышечные заболевания
Conditions that affect cortical control, brainstem coordination, peripheral neural pathways, or the neuromuscular junctions of the muscles of the swallow yield changes in deglutition. This increases the risk of penetration and aspiration.
Поражение моторных нейронов
As deterioration of the upper and lower motor neurons occur, the response of the muscles to stimuli decreases. This can affect all muscles relating to the swallow. It may present as difficulty initiating a swallow, reduced hyoid elevation, poor epiglottic Deflection, and incomplete relaxation of the UES. This might also affect the peristaltic wave, and therefore a person may show difficulty with solid consistencies earlier than liquid consistencies. As noted by Langmore, Grillone, Elackattu, and Walsh (2009), “virtually all patients experience swallowing problems at some point in the disease if they live long enough.” Prevalence of dysphagia is estimated at 81% at the time of death (Hardiman 2000).
Цереброваскулярные нарушения
Both cortical cerebrovascular accidents (CVAs) and brainstem strokes will affect swallowing. Dysphagia is present in 27–30% of persons with traumatic brain injury, and from 8.1 to 80% of those with acquired brain injury, with an average of 50% for those with stroke (Langdon et al. 2007; Takizawa et al. 2016; Ramsey et al. 2003). Presentation of dysphagia in these cohorts varies, with symptoms in stroke relating to the area of lesion. Presentation in those with TBI or executive function impairment includes nonmechanical symptoms of dysphagia, for example, overfilling the mouth and drinking/eating too quickly or slowly. A large proportion will improve spontaneously but this may be over months. Brainstem lesions tend to fair worse as it is more likely that cranial nerves IX–XII will be affected creating a combined swallowing insult. Both impaired motor (poor hyolaryngeal elevation, weak pharyngeal constriction, incomplete airway closure, uncoordinated pharyngoesophageal segment function) and sensory (inability to feel bolus and trigger airway protection and patterned swallow phases) function is found. Screening of all individuals presenting with CVA is vital. Many screening tools have been proposed including bedside assessments and questionnaires. Unfortunately, these tools will miss a significant proportion of impaired swallowers – those with silent aspiration. Instrumental assessment is the most reliable way to assess swallow safety but requires additional resources and time and is not always appropriate in the palliative care setting. Disordered deglutition post stroke is associated with worse outcomes, longer hospital stay, increased likelihood of readmission, and pneumonia within the first 3 months (Ramsey et al. 2003). Early rehabilitation can greatly enhance swallow recovery and protect against complications.
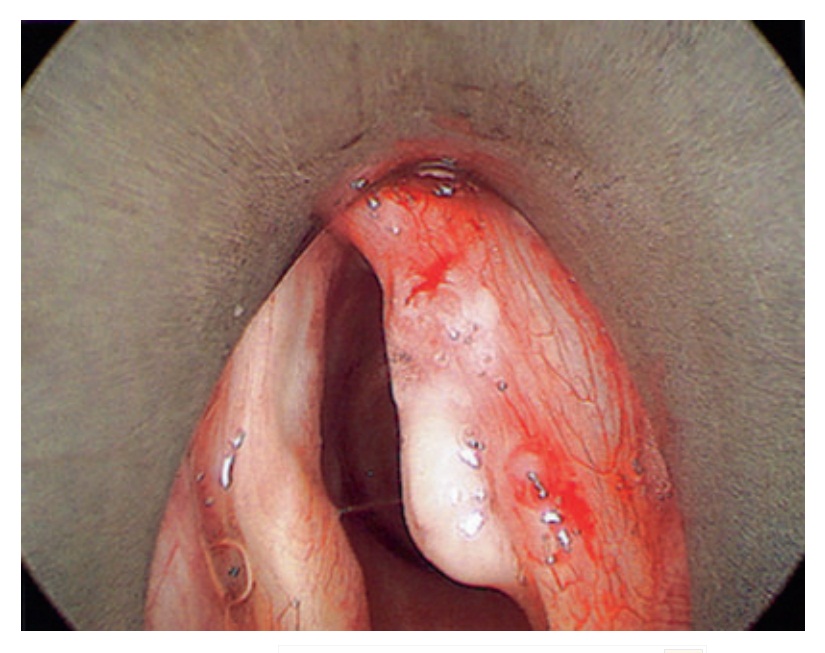
Фиг. 1. Endoscopic clinical photograph of right vocal fold squamous cell carcinoma

Фиг. 2. Endoscopic clinical photograph of right vocal fold post-resection of squamous cell carcinoma
Болезнь Паркинсона
Parkinson’s disease may affect both the motor and non-motor aspects of the swallow. It is noted that dysphagia occurs in a high proportion of people with Parkinson’s disease. This figure ranges from 33% (based on subjective bedside assessments) to 80% (based on objective assessments) (Kalf et al. 2012). Dysphagia in Parkinson’s disease is more prevalent in males; however, it is also linked with the duration of the disease and the presence of dementia (Cereda et al. 2014). As with other cohorts, dysphagia may be evident with reduced efficiency of mastication, poor base-of-tongue contraction, poor hyoid elevation, and pharyngeal residue, all of which pose a risk of penetration and aspiration.
It should be particularly noted that only a small proportion of people with dysphagia and Parkinson’s disease are able to identify symptoms of swallowing problems. This figure is estimated to be between 20 and 40% upon questioning and only 10% reporting dysphagia spontaneously (Bird et al. 1994; Suttrup and Warnecke 2016). This is significant, as pneumonia is the most common cause of death in the population of people with Parkinson’s disease (Morgante et al. 2000).
Рассеянный склероз
Oropharyngeal dysphagia occurs in over 30% of multiple sclerosis cases (Prosiegel et al. 2004). As with all other forms of oropharyngeal dysphagia, this entails risk of aspiration and penetration and subsequent pneumonia. Persons with MS are also noted to have insufficiency of velopharyngeal elevation, which leads to the presence of hyper ю/— hyponasality (Vitorino 2009). This could also increase the risk of nasal regurgitation, given the role of the soft palate in preventing bolus entry into the nasopharynx.
Деменция
It is estimated that around 45% of persons with dementia also present with dysphagia (Horner et al. 1994). Others report prevalence as high as 80% (Wasson et al. 2001) and 84% (Horner et al. 1994).
Dysphagia may present differently depending upon the particular dementia experienced (Easterling and Robbins 2008). For example, persons with Alzheimer’s disease may forget to eat, be unable to chew, forget to swallow, and show poor pharyngeal clearance (Horner et al. 1994), while persons with frontotemporal dementia may experience an increase in appetite and change in food preferences, have larger bite sizes, and eat nonedible items (Easterling and Robbins 2008). Furthermore, persons with vascular dementia show difficulty chewing and coordinating a swallow (Gallagher 2011).
Наружения, связанные с желудком
Gastroesophageal reflux disease is highly prevalent, with estimates of 14% of adults reporting weekly reflux symptoms (Eusebi et al. 2017). Therefore, a large proportion of those receiving end-of-life care will experience reflux as a preexisting comorbidity. Factors related to the terminal disease and its treatment may further impact this and increase reflux related irritation. These include medications, radiotherapy, body position, saliva production, and surgery. In patients who receive narcotic analgesia, there is a diminution of the spontaneous swallow rate, and oral dryness can be a medication side effect – a combination which can produce swallow issues. Loss of saliva increases reflux irritation due to loss of buffer, lubrication, and mechanical flushing protection. In rare cases impingement on the esophagus or gastric inlet by malignant disease may impair bolus throughput, and certainly esophageal and gastric cancer can produce marked feeding difficulties both through direct obstruction and loss of appetite.
Stomach emptying typically occurs within 90 min. Medication can slow this process and result in induced gastroparesis. Symptoms reported are early satiety and loss of appetite resulting in smaller meal volumes.
Наружения глотания, связанные со старением
As we age, our swallow naturally changes. These changes may not result in direct swallow impairment but may reduce physiological reserve when the swallow is challenged. This has been termed “presbyphagia” to indicate the relationship to the aging process.
Logemann et al. (2013) noted significant changes in the swallow, including a change in the method of propelling the bolus from the front to the back of the mouth increasing duration of the oral stage. There is also a delay in the triggering of the swallow reflex (and subsequent pharyngeal phase), with a delay in the hyoid and larynx returning to the pre-swallow position (older persons have a slower refractory period). An increased number of penetration events are also noted, but in the absence of aspiration. In a recent normative study, quantitative oropharyngeal and esophageal swallowing parameters remained fairly constant in those up to 80 years after which there was an appreciable increase in oropharyngeal and esophageal transit times and reduction in pharyngeal constriction (strength) (Miles et al. 2016).
Summary
Swallowing problems are present in many individuals nearing their end-of-life with wide-ranging and overlapping causes but are poorly reported and subsequently may not receive enough attention. Swallowing comprises a series of interconnected phases, whereby disruption can occur at any stage. The greatest risk of swallow impairment is penetration and aspiration with development of chest infection and its associated mortality.
Dysphagia can occur in any person, whether it is part of the natural aging process, brain injury, or a neuromuscular disorder. Given the complex nature of deglutition, we recommend that people who are deemed at risk of dysphagia by a health professional (due to diagnosis or reported symptoms) should undergo swallow screening and assessment. This would then determine the need for diagnostic assessment. This will be further expanded in the next section.
Оценка
In order to prevent the consequences of swallowing problems, it is crucial to be able to identify those at risk or with impairments who need expert management. Complete swallow assessment is time-consuming, so initial screening tools have been proposed to allow a more targeted approach.
Скрининг
Screening for swallowing problems is recognized as the first part of a holistic swallow assessment. Screening can usually be completed by any health professional and is mandatory within 24 h in some locations for all people who have had a stroke (Cichero et al. 2012).
Screening is not intended as a substitute for a comprehensive assessment. It is intended to identify symptoms of dysphagia and signs of swallow impairment and therefore identify those who may require comprehensive assessment by a speech pathologist (Martino et al. 2009).
Various screening tools are available within the literature. Broadly speaking, these cover areas which can be examined by any health professional (Cichero et al. 2009; Martino et al. 2009; Stewart 2003). Speech Pathology Australia, the Australian SP representative body, suggest the inclusion of:
- Diagnosis: Determining if the person’s diagnosis heightens their risk of dysphagia (e.g., stroke, neurodegenerative condition, head and neck cancer).
- Interview: This involves discussing the person’s history of swallowing, presenting symptoms (e.g., frequency of overt signs of aspiration, chest infections, feelings of food being stuck).
- Symptoms: Symptoms of slurred speech, facial weakness, drooling, coughing on saliva, weak/ absent cough (note: gag reflex is not a predictor of swallowing impairment and is excluded from screening) (Martino et al. 2009).
- Sip test: Where appropriate, patients are given a sip of thin water, and observations are made for coughing, throat clearing, change in voice quality, drooling, and change in respiration. Some debate exists about the validity of water-sip tests, but screening with water-sip tests where appropriate is still recommended in the Speech Pathology Australia Dysphagia Guideline (Cichero et al. 2012; Osawa et al. 2013).
Various local health districts have designed their own adapted version of these screening tools; however, the content remains largely similar. Screening tools are also part of the usual admission process for residential aged care facilities (both highand low-level care) and often form part of acute-care policy for initial admission screening.
Those who “fail” the screening (i.e., demonstrate signs suggestive of aspiration or other risk factors for swallowing problems) are referred to speech pathology for comprehensive assessment of swallowing, leading to accurate diagnosis and targeted management.
Всесторонняя оценка
Comprehensive assessment of dysphagia most commonly begins with a functional assessment. This involves an extensive case history, cranial nerve examination, oromotor examination, and food/fluid trial (where appropriate) at the bedside. Further assessment with instrumental measures such as fibreoptic endoscopic evaluation of swallowing (FEES) or videofluoroscopic swallowing studies (VFSS) may be performed although this is not always possible in every setting.
Функциональная
A speech pathologist begins examination with an extensive case history. Examination of the medical file, previous SP intervention, comorbidities and medications, current diet/fluids, palliative care phase/PCOC stage, concerns about eating, and recent chest history are reviewed. Preliminary hypotheses are then formed about a person’s likely presentation.
Прикроватная оценка
The first part of the assessment is determining if a more in-depth assessment is warranted.
Patient presentation
First and foremost, a patient must be sufficiently alert for assessment. It would be inappropriate to attempt an assessment on a person who is delirious, unable to maintain eye contact, and unable to stay awake to sufficiently swallow a bolus (e.g., 2 min). The lack of alertness will make assessment unreliable and increase risk of aspiration. The decision must be made for each individual by the supervising clinician. Similarly, it may be inappropriate to perform a full assessment on someone with severe pain, and assessment may need to be deferred until pain is controlled.
A patient is observed for their ability to manage their own secretions, the presence of tubes (e. g., nasogastric, nasojejunal, tracheostomy), and their ability to cooperate with an assessment (Cichero et al. 2012).
- Cranial nerve examination/oromotor examination
To begin the physical examination, the speech pathologist observes the oral cavity. Assessment includes the health of the oral mucosa (e.g., pink), moisture, health of the soft tissue (e.g., presence of oral thrush), and dentition (presence of dentures/own dentition, cleanliness of teeth). The odor of the mouth may be observed, and quality of the secretions (e.g., stringy, clear), as well as the pre-existing anatomy of the oral cavity.
A cranial nerve examination will assess those nerves innervating muscles throughout the swallow. The speech pathologist will request the patient to perform a series of movements and will observe symmetry, deviation, range/rate of movements, strength of movements, and sensation. If the patient is unable to follow instructions, the speech pathologist may complete the movements in front of the patient so that they may copy their movements.
- CNV – observe the jaw for range of lateral and opening/closing movements, strength of jaw, strength of masseters, and mandibular sensation, tongue sensation, and taste.
- CNVII – observe the face for closure at rest, droop, sensation to upper face, lip rounding, lip speed during “oo-ee” said in quick, repetitive succession, strength of facial movements, and lip retraction.
- CNIX and CNX – observe the soft palate for symmetry of elevation, and observe nasal emission, voice quality, pitch variation, volume control, and respiration.
- CNXII – examine tongue movement, range and strength of movement, fasciculations, weakness, or atrophy.
- Observe a volitional cough for strength and productivity. Cough reflex testing may also be performed at bedside in some settings (see below).
- Observe and palpate the hyoid for dry swallow strength and perceived range of movement.
- The gag reflex is no longer consistently recommended as part of the assessment as it is innervated by a different nerve than the larynx and pharynx and does not have predictive value (Cichero and Murdoch 2006).
It should be noted that not all parts of the assessment will always be possible. In palliative care, a patient may be highly distressed and only able to tolerate small amounts of oral intake or remain alert for very short periods. In this case, a cranial nerve examination may not be possible in a formal, structured way, and priority may be given to ensuring the person’s comfort.
Food/fluid trial
If deemed appropriate, the speech pathologist may commence a food and/or fluid trial to determine the most appropriate recommendations.
Positioning
Ideally, a person would be positioned upright for assessment. Recent evidence suggests that sitting at 90 degrees upright may not be best for those with poor sitting balance or trunk control, as this places undue pressure on their respiration, and that 45 degrees is adequate for this population (Park et al. 2013).
Self-feeding
It is most useful to assess a person as close to their natural abilities as possible, particularly if the person is at home or will return home. It is important to determine if the person feeds themselves and if they use any prescribed or specific cutlery or other utensils.
To begin, the speech pathologist instructs the subject to take a single bite or mouthful of drink or food. The attending speech pathologist will determine if the food and fluids should be those which the person is already receiving or the safest oral diet (e.g., puree and extremely thick, in most cases).
As the person consumes the food or fluids, observations are made for the oral preparatory/ oral phase and the pharyngeal phase. The esophageal phase is not assessed at bedside.
Oral phase
Observation of the presence of any anterior spillage, oral pooling, bolus control, mastication, oral transit time, and anterior-posterior movement of the bolus is made.
Pharyngeal phase
Pharyngeal phase: observation of the timing of the swallow, effort required for the swallow, laryngeal movement, voice quality post swallow, coughing, throat clearing, nasal regurgitation, and swallows per bolus are documented. Aspiration may be suspected based on cough response, cervical auscultation, or change in oxygen saturations. Laryngeal movement is not objectively assessed at bedside, but is assessed via palpating the hyoid during the swallow. Assessment of timing of a swallow is not possible at bedside, as the true location of the bolus for swallowing is unknown. At bedside, the speech pathologist may also try compensatory strategies with the food and fluids, which may allow different recommendations to be made.
In many cases, a bedside examination is all that is available to a speech pathologist for assessment. Though objective measures are considered the “gold standard,” these are not necessarily appropriate for someone receiving palliative care or for someone in a subacute facility. It may cause them unnecessary inconvenience, may tire them, and may yield no changes to their management.
Инструментальная
Additional ancillary assessments may be considered on a case-by-case basis.
Cervical auscultation
As part of a comprehensive assessment, a speech pathologist may utilize cervical auscultation to provide aural information about the swallow. It is not suiтаблица as a stand-alone assessment, but can be useful for supporting hypotheses as an adjunct to additional assessments. Cichero and Murdoch (2006) provide an exemplary summary of this method.
Cervical auscultation utilizes a standard stethoscope. Some speech pathologists prefer pediatric stethoscopes, as they better fit under the neck with a firm seal and account for additional skin surface area.
The speech pathologist places the stethoscope with a firm seal, just below the cricoid cartilage and in a central location (Hirano et al. 2001). The patient performs a swallow of saliva, and the speech pathologist observes the normal sounds of the swallow. Usually, two clicks signify (1) the closure of the laryngeal valve and the projection of the base of the tongue to the posterior pharyngeal wall and (2) the opening of the upper esophageal sphincter and the pharyngeal stripping wave (Cichero and Murdoch 2006). Pharyngeal residue may be audible, as well as multiple swallows per bolus. The sounds of swallowing become longer as we age, and this may be perceived by the ear (Cichero and Murdoch 2006). During the observation of quiet respiration, the speech pathologist can often hear the clarity of the breath sounds and if there is any fluid, food, or saliva sitting in the airway.
Pulse oximetry
The measure of oxygen saturation is another useful adjunct to assessment. Again, this is not suiтаблица for use as a stand-alone assessment but provides useful information about suspected aspiration.
To utilize pulse oximetry, a pulse oximeter is placed on the person’s index finger while a food and fluid trial takes place. The baseline oxygen saturation is observed, as well as the usual fluctuations in saturation for that person. The value is observed for change during assessment, and a drop of 2% is significant for indicating the presence of aspiration (Lim et al. 2001). However, most devices have a 2% error rate, so information must be used in conjunction with other clinical indicators to determine the outcome of assessment. As oxygen saturation does not drop immediately at the time of aspiration, monitoring of saturation is necessary for several minutes after the trial.
Cough reflex testing
Current research is analyzing the effectiveness of screening patients in acute care utilizing cough reflex testing to assist with identification of silent aspiration at bedside. This is completed with a standard nebulizer mask with free-flow output of
8 L and restricted flow of 6.6 L/min and 0.4–0.8 mol/L of citric acid (Miles et al. 2013). This assists with identification of silent aspiration at bedside. This is useful as an adjunct to assessment to assist with identification of those at risk of silent aspiration. However, the application of this measure may not be appropriate in all settings, particularly in palliative care.
Manometry and electromyography
Manometry can be used to examine the pharyngeal contraction during a swallow and is conducted by a gastroenterologist. A small tube is passed through the nasal passage into the esophagus. Electromyography is performed with electrodes placed on the skin over muscles of interest or directly into the muscles themselves (Cichero and Murdoch 2006).
With these measures, pharyngeal and esophageal contractions are measured for effectiveness. Again, this may be a useful adjunct for some assessments, but application in palliative care and subacute settings is limited due to the absence of radiological machines and the patient status (i. e., invasive investigations may not be appropriate).
Fibreoptic endoscopic evaluation of swallowing (FEES)
Fibreoptic endoscopic evaluation of swallowing (FEES) is an instrumental evaluation technique that can be performed at bedside but requires specialized equipment and a trained clinician familiar with endoscopy. Also known as functional endoscopic evaluation of swallow, this involves the insertion of a flexible endoscope (fiberoptic or videoscope) passed per naris Assessment is made of anatomy, function, secretions, and sensation alongside evaluation of penetration, aspiration, and regurgitation when the subject is given food or fluid or swallows saliva. The study can be tailored to the individual’s needs and abilities, and the clinician must have clear goals for the study and appreciate the anatomy and function of the laryngopharynx when performing this evaluation.
FEES can provide invaluable information about laterality of deficits, secretion management, compensatory strategies, and swallow safety. It is quick to perform and usually well tolerated. There is no exposure to ionizing radiation and FEES may be performed in both adults and children. Impediments to utilizing FEES include the cost of equipment and need for training, patient discomfort and nasal access issues, difficulty in performing in those with poor cognition, “whiteout” effect wherein the view is obscured middeglutition as the pharynx constricts around the endoscope, and ability to clean and disinfect endoscopic equipment. FEES may also be used as a training tool through biofeedback wherein the patient can watch the video screen and practice maneuvers that alter swallows. Examination can be recorded to video files allowing review at leisure and by clinicians not present during the performance of the study.

Фиг. 3. Lateral fluoroscopic view mid-swallow demonstrating retroflexed epiglottis outlined by barium bolus with hyoid elevation and closed airway anteriorly and bolus flowing through the open pharyngoesophageal segment posteriorly
With the advent of efficient battery-powered endoscopes, FEES is more accessible than previously and may represent the instrumental evaluation that is easiest to achieve in a palliative setting, given the difficulty in moving ill patients to radiology suites for videofluoroscopic evaluations.
Videofluoroscopic swallow study (VFSS – Also Known as Modified Barium Swallow, MBS)
VFSS (previously termed modified barium swallow – MBS) is a dynamic, real-time videofluoroscopic swallow evaluation. Ionizing radiation is administered to obtain images through a fluoroscope, and therefore this must be performed in a radiological facility by a speech pathologist or medical radiation technologist ю/— a radiologist (Figs. 3 and 4).
The VFSS utilizes barium sulfate (either powder or liquid) as a contrast agent which is then mixed with food and fluids.
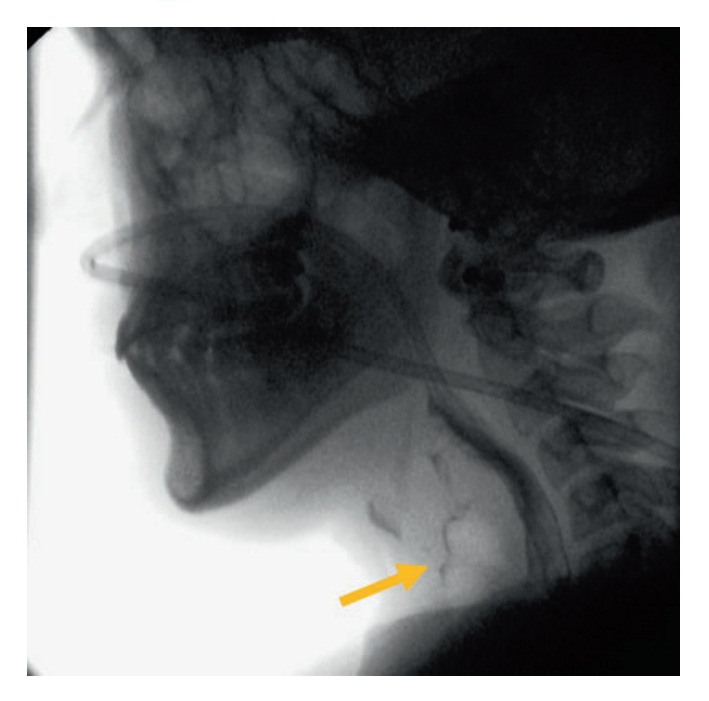
Фиг. 4. Lateral fluoroscopic view mid-swallow demonstrating aspiration of bolus into the airway anteriorly (short arrow) with majority of the bolus passing posteriorly through the pharyngoesophageal segment. Note a nasogastric tube is in situ
The patient is positioned upright for assessment, and contrast is consumed. On VFSS, the following areas are visible (in addition to those seen at bedside):
- Base of the tongue to the posterior pharyngeal wall contraction
- Bolus cohesion
- Soft palate retraction and elevation
- Contraction of the pharyngeal constrictors
- Deflection of the epiglottis (Фиг. 3)
- Elevation and anterior movement of the hyoid
- Opening of the upper esophageal sphincter
- Passage of the bolus through the pharynx to the esophagus and stomach
- Airway violation (aspiration) (Фиг. 4)
- Residue or bolus oscillation
On VFSS, some anatomic abnormalities may be observable. These include osteophytes, diverticula, and cricopharyngeal bar. VFSS provides an excellent overview of the swallowing function from the oral cavity to stomach. It delivers a raft of information which can inform management and allows testing of any potential rehabilitation and compensatory strategies, as well as the safest food and fluid consistencies. By using quantitative measures, further data may be extracted from VFSS (Leonard and Kendall 2014; Leonard et al. 2000; Miles et al. 2015, 2016) and compared to well-documented normative data for age and gender. As exposure to ionizing radiation occurs, it behoves the clinician to maximize the value of the study. VFSS should not be considered a binary test of aspiration alone.
Вмешательства
The combination of functional and instrumental assessments allows the multidisciplinary team to make recommendations for diet and management. The SP provides recommendations regarding the safest oral diet (if any), environmental support, behavioral strategies, and rehabilitation and may support discussions regarding choice making for quality of life. Otolaryngologists, physicians, oncologists, and supervising medical personnel can provide additional advice regarding surgical options and pharmacological treatments.
Нефармакологические
Non-pharmacological interventions are most commonly guided by a speech pathologist. These vary from compensatory techniques (behavioral strategies, food/fluid modification, environmental modification, and symptom management) to rehabilitative exercises and to a quality-of-life approach which places the emphasis on choice-making.
Компенсационные
- Behavioral strategies
Behavioral strategies are those designed to optimize the safest oral diet by teaching a person to perform an action before, during, or after a swallow. It should be noted that these may be conservatively used in palliative care, and only under the direction of a speech pathologist and medical team. This might involve the following:
- Head tilt/rotation – tilting the head or turning it to the side of weakness, directing the bolus to the stronger side (Logemann 1998; Logemann et al. 1989).
- Chin tuck – tucking the chin in toward the chest to widen the pharyngeal space (Logemann 1998).
- Body posture – this may involve side lying to the stronger side, allowing a person to swallow and clear residue with the aid of gravity (Sura et al. 2012).
- Supraglottic swallow – a swallow is performed followed by an immediate cough; the cough is not preceded by breathing in (intended to clear material in the airway) (Martin et al. 1993).
- Super-supraglottic swallow – a supraglottic swallow is performed with the addition of an effortful swallow (Martin et al. 1993).
- Multiple swallows – several swallows to ensure clearance of a bolus and any remaining pharyngeal residue.
- Effortful swallow – swallowing as hard as you can to increase hyolaryngeal excursion, oral and pharyngeal pressures, and subsequent movements (Kahrilas et al. 1991).
This is not an exhaustive list of all behavioral strategies, but those most commonly utilized and those that may apply to the palliative care setting. More rigorous approaches (such as neuromuscular electrical stimulation – NMES) are not appropriate for the palliative care setting (Xia et al. 2011).
Модификация пищи/жидкости
Food and fluid modification is designed to ensure a person is able to adequately manipulate, form, and swallow a bolus with minimal residue and minimal entry into the airway. Thicker viscosities may slow the bolus transportation; softer foods make it easier to chew or moister to allow for better cohesion.
The names and definitions of food textures vary across the globe; however, they generally involve:
- Full/normal – unmodified food.
- Soft/texture A – food with lumps up to 1.5 cm in size. Food should be well cooked, moist, and fork mashable (some networks break this further into two levels of soft diets).
- Minced/texture B – has small lumps (up to 0.5 mm), which are moist and soft. Lumps should be tongue mashable.
- Puree/texture C – smooth, lump-free food that holds its shape on a spoon.
Консистенции жидкости также различаются, но обычно они:
- Thin/normal – unmodified fluids
- Mildly thick/level 150/nectar/level 1 – runs through the prongs of a fork in a continuous stream
- Moderately thick/level 400/honey/level 2 – runs through the prongs of a fork slowly in large drips
- Extremely thick/level 900/pudding/level 3 – sits on a fork and does not run through
Given the numerous labels of each classification, as well as the variety across the world, Cichero et al. (2013) sought to develop standardized classification for terminology. The “International Dysphagia Diet Standardisation Initiative” (IDDSI) recommends a total of ten textures, on a continuum labeled from 0 to 7. Most noticeably, there has been a suggested addition of “slightly thick” and “liquidized” diet. Slightly thick refers to fluids which naturally occur and are thicker than normal fluids, and liquidized foods refer to smooth, lump-free and liquid food (moderately thick). The latter is a very useful diet in palliative care (as well as in other settings), particularly for those that prefer drinking to eating (such as those with MND, dementia, or esophageal cancers).
There remains a great variety in the application of recommended diets. Standardization is not yet mandatory, and communication between speech pathologists is vital to prevent incorrect diet and fluid provision. This standardization project shows great promise for improving the continuity of modified diets and fluids across the world, reducing confusion, and improving quality of life for patients.
Протокол свободной воды
A speech pathologist may recommend modified diet and fluids, but may also make suggestions for a “free-water protocol” to assist with hydration and maintenance of quality of life.
The Frazier Free Water Protocol involves the provision of as much thin water as desired to a patient (Panther 2005). Criteria for recommended use of this protocol include nil excessive choking and subsequent physical stress, waiting for 30 min after a meal, use of any recommended swallowing strategies, diligent oral care, medications with thickened fluids/fruit puree, and family education. Karagiannis and Karagiannis (2014) provided additional criteria of good mobility and cognition, resulting in nil significant increase in rates of aspiration pneumonia and significantly improved quality of life of participants. Research remains inconclusive about the overall effectiveness in increasing hydration, and implementation should be at the discretion of the supervising clinicians.
Модификация среды
In addition to the above strategies, environmental modification may be utilized to aid safe swallowing without requiring the person to change their behavior. Environmental modification may involve altering the method of feeding, distractions, and the local environment. For example:
- Use of a straw or spout cup – moderates the bolus flow and amount and assists with poor upper limb control.
- Use of adapted cups (e.g., cutout cup) – moderating the amount that can flow from the cup with the maximum tipping of the cup.
- Use of finger foods – useful for those who eat well with their hands but not with cutlery or other utensils.
- Reduction of local distractions – ensuring a person is focused on their meal by turning off the television and reducing the number of people in the room (Kyle 2012).
- Eating with the patient to provide a model of eating behavior (also often useful for those with dementia).
- Providing six smaller meals instead of three large meals to reduce the burden of eating large meals.
- Dentition may affect texture choices. Ill-fitting dental appliances can be an impediment and cause xerostomia. Loss of dentition may make eating solid foods impossible, and input of a dentist may help in some cases.
- Lubrication is crucial to deglutition. Saliva helps form the bolus, initiates digestion, protects the mucosal lining and dentition, provides antibodies and antibacterial molecules, mechanically flushes oral and pharyngeal spaces, and dissolves tastants in food. Xerostomia is a common consequence of disease treatment, particularly radiotherapy and medications. It must be considered and compensated as far as possible with introduced hydration, minimizing drying medications, moisturizing food, and meticulous dental hygiene (see “Oral Care” chapter).
Стратегии питания
Feeding strategies may be useful with presenting symptoms. These can be implemented by carers and professionals and for those persons who require full assistance with feeding. As always, a person should only be fed when they are alert and able to swallow.
- Alternate between food and fluids to assist with washing away oral and pharyngeal residue.
- Alternate between tastes, textures, and temperatures (Easterling and Robbins 2008).
- Alternate between bolus and an empty spoon – light pressure on the tongue with the spoon may assist with initiating a swallow.
- Gentle touch may increase nutritional intake and attention to the meal (Eaton et al. 1987).
- Discuss the food taste, smell, and color (Wasson et al. 2001).
Independence is always encouraged and, for the most part, yields better swallowing. However, feeding strategies assist with swallow stimulation for someone whose initiation is compromised.
Симптоматическое лечение
In some cases, it may be appropriate to recommend rehabilitative exercises to improve the function of a swallow. These are rarely used in palliative care and are most commonly recommended in a rehabilitation setting.
Восстановительное
- Masako – this involves placing the tongue between the upper and lower teeth and holding it in position while swallowing. The Masako improves the base of the tongue to posterior pharyngeal wall contraction (Fujiu and Logemann 1996).
- Mendelsohn – this involves raising the larynx and consciously inhibiting the lowering of it for a sustained period. The Mendelsohn assists with opening the upper esophageal sphincter (McCullough et al. 2012; Mendelsohn and McConnel 1987).
- Shaker – this involves lying in supine, keeping the shoulders on the bed, and lifting the head to look at the toes, thereby improving the anterior movement of the larynx and the opening of the UES (Shaker et al. 1997).
Выбор решений
Above all, the primary consideration of the speech pathologist for the person receiving palliative care is their ability to choose what they wish. A speech pathologist may make recommendations which are believed to be the safest based on available evidence, but the person may at any time elect their preferred diet and fluids.
At the forefront in all forms of healthcare is the necessity to treat each person as an individual. A person may wish to accept the risks and consequences of aspiration, or they may wish to take a more conservative approach. They may choose to have a combination of approaches or implement the Frazier Free Water Protocol.
It is the role of the speech pathologist and the multidisciplinary team to educate the patient and family, ensure they feel able to make a choice, and assist them in carrying out their wishes to ensure their quality of life is maximized.
Фармакологический
Medication is part of holistic care of the terminal patient. They may require analgesics and regular medication for comorbid conditions. In addition they may receive treatment-specific medication such as chemotherapy. All medications may have side effects, some of which can impair deglutition. As mentioned, drying of the pharyngeal lining impairs swallow and can result in pain or opportunistic infection, particularly Candida spp. Careful consideration should be given to current medications and their risk benefit profile, as well as any new proposed or introduced medications.
Pharmacological therapies that may offer benefit for swallow disorders include lubricants (artificial saliva, lozenges (sugar-free), gum (sugarfree), and alginates), antacids (H2 blockers, proton pump inhibitors, binders/alginates), analgesics which allow swallow without pain, prokinetic agents (domperidone, metoclopramide, erythromycin) which enhance esophageal function and help secretion control, anti-inflammatories (nonsteroidal and steroidal), and antifungals. Each medicine should be carefully evaluated and considered in conjunction with other current medicines to avoid drug interactions. There are no specific medications to “treat dysphagia,” and use of medicines will be determined by symptoms and comorbid disease. Rarely should drying medications be used as these typically result in thickening of sputum and make it more difficult to clear. In specific cases amitriptyline given at 10 mg nocte may reduce overall saliva production without severe side effects, but all medications should be discussed with the supervising clinicians.
Хирургический
Rarely is surgery appropriate in palliative care, although a terminal illness is not a complete contraindication to targeted surgery. Again, each individual case will require appropriate consideration.
For example, airway protective procedures such as injection laryngoplasty or medialization may be very helpful in preventing choking and aspiration. This improves quality of life and communication during a patient’s final days. For some, an injection laryngoplasty may be performed in the clinic under local anesthetic, obviating the need for general anesthesia and speeding recovery. There are several other procedures to protect the airway such as laryngeal framework surgery (arytenoid adduction), tracheostomy, laryngeal suspension, or laryngotracheal separation; however, these are increasingly invasive and often not appropriate in a palliative care setting.
Dilatation of aerodigestive sphincters, strictures, rings, or bars may be appropriate as these can often be accomplished under local anesthetic or sedation and may improve bolus transit and reduce residue that predisposes to airway violation. Feeding tube placement such as percutaneous endoscopic gastrostomy (PEG) may be undertaken during treatment and in place when patients transition to palliative care. These can be invaluable as a medication delivery system and to provide adequate hydration but have not been shown to significantly reduce mortality associated with aspiration. Nutritional supplementation can also be provided but must be undertaken after careful discussion with a dietitian and palliative care consultant. Cricopharyngeal myotomy or botulinum toxin injection of the cricopharyngeus muscle may improve swallow in patients with obstructive pharyngoesophageal segments. Onabotulinum toxin can also be utilized for laryngeal spasm or cervical dystonia and has been used to reduce saliva output from the submandibular glands in some cases.
Заключение
When an individual is approaching the end of their life and is aware of a terminal diagnosis, the ability to communicate and to be part of social events with family and friends is of the utmost importance. Nearly all social situations include eating, and when this is impaired, there can be a significant decrement in the quality of life for the individual. Many patients will experience swallowing difficulties as a result of their disease process, the treatment of the disease whether it be surgical or medical, and of increasing frailty and deconditioning. Palliative care teams should be aware of the high prevalence of swallow impairment and should directly inquire about symptoms and observe for signs of disordered deglutition. Early and frequent assessment by speech pathologists can provide a marked improvement in swallow function and reduction in risk associated with poor swallowing. Limiting pulmonary soiling is vital in maintaining health, and a competent swallow is at the heart of this.
Simple dietary and behavioral measures can be employed to facilitate swallowing. Careful medication management will minimize xerostomia and reduce pain. Targeted surgical procedures may minimize airway violation or treat strictures. Non-oral feeding can maintain nutrition but deprives the individual of the pleasure of food. Even taking a small amount of oral diet or liquid for pleasure should be considered, particularly in the terminally ill. At times, the risk of aspiration may be accepted where the overall quality of life gain from eating outweighs the swallow risk – a decision that only the patient can make. The most important approach is to involve all team members and the patient to individualize the treatment plan so that each patient receives the options most suited to their needs. Education of the individual and their family around safe eating practices, food preparation, and compensatory strategies can go a long way to mitigating swallow risks. It also empowers the patient and their support network allowing them to plan day-to-day life. At all times the patients’ wishes and preferences must be at the core of recommendations and management strategies.
Литература
Allen J. Cricopharyngeal function or dysfunction – what’s the deal? Curr Opin Otolaryngol Head Neck Surg. 2016;24:494–9.
Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing. 1994;23(3):251.
Bonilha H, Simpson A, Ellis C, Mauldin P, Martin-Harris B, Simpson K. The one-year attribuтаблица cost of post-stroke dysphagia. Dedicated to advancing the art and science of deglutology. Dysphagia. 2014;29(5):545–52. https://doi. org/10.1007/s00455-014-9543-8.
Cereda E, Cilia R, Klersy C, Canesi M, Zecchinelli A, Mariani C, et al. Swallowing disturbances in Parkinson’s disease: a multivariate analysis of contributing factors. Parkinsonism Relat Disord. 2014;20(12):1 382–7. https://doi.org/10.1016/j.parkreldis.2014.09.031.
Cichero J, Murdoch B. Dysphagia: foundation, theory and practice. Chichester: Wiley; 2006.
Cichero JA, Heaton S, Bassett L. Triaging dysphagia: nurse screening for dysphagia in an acute hospital. J Clin Nurs. 2009;18(11):1649–59. https://doi.org/ 10.1111/j.1365-2702.2009.02797.x.
Cichero J, Baldac S, et al. Clinical guideline: dysphagia. Melbourne: Speech Pathology Australia; 2012.
Cichero J, Steele C, Duivestein J, Clavй P, Chen J, Kayashita J, et al. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Rep. 2013;1(4):280–91. https://doi.org/ 10.1007/s40141-013-0024-z.
Drake RL, Vogl AW, Mitchell AWM. Gray’s anatomy for students. 2nd ed. Philadelphia: Churchill Livingstone/ Elsevier; 2010.
Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nurs. 2008;29(4):275–85. https://doi.org/ 10.1016/j.gerinurse.2007.10.015.
Eaton M, Mitchell-Bonair I, Friedman E. The effects of touch on nutritional intake of chronic organic brain syndrome patients. Alzheimer Dis Assoc Disord. 1987;1(2):107–8. https://doi.org/10.1097/00002093-198701020-00020.
Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2017. https://doi.org/10.1136/ gutjnl-2016-313589.
Fujiu M, Logemann JA. Effect of a tongue-holding maneuver on posterior pharyngeal wall movement during deglutition. Am J Speech Lang Pathol. 1996;5(1):23. https://doi.org/10.1044/1058-0360.0501.23.
Gallagher R. Swallowing difficulties: a prognostic signpost. Can Fam Physician. 2011;57(12):1407.
Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(29):3235. https://doi.org/ 10.1200/JCO.2015.61.6995.
Hardiman O. Symptomatic treatment of respiratory and nutritional failure in amyotrophic lateral sclerosis. J Neurol. 2000;247(4):245–51. https://doi.org/10.1007/s004150050578.
Hirano K, Takahashi K, Uyama R, Michi K-I. Evaluation of accuracy of cervical auscultation for clinical assessment of dysphagia. Jpn J Oral Maxillofac Surg. 2001;47(2):93–100. https://doi.org/10.5794/jjoms.47.93.
Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Disord. 1994;8(3):177.
Kahrilas PJ, Logemann JA, Krugler C, Flanagan E. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Phys. 1991;260(3 Pt 1):G450–6.
Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311–5. https://doi.org/10.1016/j. parkreldis.2011.11.006.
Karagiannis M, Karagiannis TC. Oropharyngeal dysphagia, free water protocol and quality of life: an update from a prospective clinical trial. Hell J Nucl Med. 2014;17 Suppl 1:26.
Kyle G. Medication management in older people with dementia. J Community Nurs. 2012;26(1):31–4.
Langdon PC, Lee AH, Binns CW. Dysphagia in acute ischaemic stroke: severity, recovery and relationship to stroke subtype. J Clin Neurosci. 2007;14(7):630–4. https://doi.org/10.1016/j.jocn.2006.04.009.
Langmore SE, Grillone G, Elackattu A, Walsh M. Disorders of swallowing: palliative care. Otolaryngol Clin N Am. 2009;42(1):87–105. https://doi.org/10.1016/j. otc.2008.09.005.
Leonard R, Kendall K. In: Leonard R, Kendall KA, editors. Dysphagia assessment and treatment planning: a team approach. 3rd ed. San Diego: Plural Publishing; 2014. Leonard RJ, Kendall KA, McKenzie S, Gonзalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(3):146–52. https://doi.org/10.1007/ s004550010017.
Lim SHB, Lieu PK, Phua SY, Seshadri R, Venketasubramanian N, Lee SH, Choo PWJ. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16(1):1–6. https://doi.org/10.1007/ s004550000038.
Logemann JA. In: Logemann JA, editor. Evaluation and treatment of swallowing disorders. 2nd ed. Austin: PRO-ED; 1998.
Logemann JA, Kahrilas PJ, Kobara M, Vakil NB. The benefit of head rotation on pharyngoesophageal dysphagia. Arch Phys Med Rehabil. 1989;70(10):767.
Logemann J, Curro F, Pauloski B, Gensler G. Aging effects on oropharyngeal swallow and the role of dental care in oropharyngeal dysphagia. Oral Dis. 2013;19(8):733–7. https://doi.org/10.1111/odi.12104.
Martin B, Logemann J, Shaker R, Dodds W. Normal laryngeal valving patterns during three breath-hold maneuvers: a pilot investigation. Dysphagia. 1993; 8(1):11–20. https://doi.org/10.1007/BF01351472.
Martino R, Silver F, Teasell R, Bayley M, Nicholson G, Streiner DL, Diamant NE. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555. https:// doi.org/10.1161/STROKEAHA.107.510370.
Masterson L, Moualed D, Liu ZW, Howard JE, Dwivedi RC, Tysome JR, Benson R, Sterling JC, Sudhoff H, Jani P, Goon PK. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and metaanalysis of current clinical trials. Eur J Cancer. 2014;50:2636–48.
McCullough GH, Kamarunas E, Mann GC, Schmidley JW, Robbins JA, Crary MA. Effects of Mendelsohn maneuver on measures of swallowing duration post stroke. Top Stroke Rehabil. 2012;19(3):234–43. https://doi. org/10.1310/tsr1903-234.
Mendelsohn MS, McConnel FM. Function in the pharyngoesophageal segment. Laryngoscope. 1987; 97(4):483–9.
Miles A, Moore S, McFarlane M, Lee F, Allen J, Huckabee M-L. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav. 2013;118:25.
Miles A, McMillan J, Ward K, Allen J. Esophageal visualization as an adjunct to the videofluoroscopic study of swallowing. Otolaryngol Head Neck Surg. 2015;152(3):488–93. https://doi.org/10.1177/0194599814565599.
Miles A, Clark S, Jardine M, Allen J. Esophageal swallowing timing measures in healthy adults during videofluoroscopy. Ann Otol Rhinol Laryngol. 2016;125(9):764–9. https://doi.org/10.1177/0003489416653410.
Mirghani H, Amen F, Blanchard P, Moreau F, Guigay J, Hartl DM, Lacau St Guily J. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer. 2015;136:1494–503.
Moore KL. In: Moore KL, Dalley II AF, Agur AMR, editors. Clinically oriented anatomy. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2010.
Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, et al. Parkinson disease survival: a population-based study. Arch Neurol. 2000;57(4):507–12. https://doi.org/10.1001/archneur.57.4.507.
Osawa A, Maeshima S, Tanahashi N. Water-swallowing test: screening for aspiration in stroke patients. Cerebrovasc Dis. 2013;35(3):276–81. https://doi.org/ 10.1159/000348683.
Panther KM. The Frazier free water protocol. Perspect Swallow Swallow Disord (Dysphagia). 2005; 13(4):4–9.
Park B-H, Seo J-H, Ko M-H, Park S-H. Effect of 45? reclining sitting posture on swallowing in patients with dysphagia. Yonsei Med J. 2013;54(5):1137. https://doi.org/10.3349/ymj.2013.54.5.1137.
Prosiegel M, Schelling A, Wagner-Sonntag E. Dysphagia and multiple sclerosis. Int MS J. 2004;11(1):22–31.
Ramsey JC, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34:1252–7.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11(2):93–8.
Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG, et al. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. Am J Phys. 1997;272(6 Pt 1):G1518–22.
Shaw SM, Martino R. The normal swallow. Otolaryngol Clin N Am. 2013;46(6):937–56. https://doi.org/ 10.1016/j.otc.2013.09.006.
Stewart L. Development of the nutrition and swallowing checklist, a screening tool for nutrition risk and swallowing risk in people with intellectual disability. J Intellect Dev Disabil. 2003;28(2):171–87. https://doi. org/10.1080/1366825031000106945.
Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287.
Sutherland J, Hamm J, Hatcher J. Adjusting case mix payment amounts for inaccurately reported comorbidity data. Health Care Manag Sci. 2010;13(1):65–73. https://doi.org/10.1007/s10729-009-9112-0.
Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dedicated to advancing the art and science of deglutology. Dysphagia. 2016;31(1):24–32. https:// doi.org/10.1007/s00455-015-9671-9.
Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dedicated to advancing the art and science of deglutology. Dysphagia. 2016;31(3):434–41. https://doi.org/10.1007/ s00455-016-9695-9.
Vitorino J. Velopharyngeal function in adult speakers of Portuguese diagnosed with multiple sclerosis. NeuroRehabilitation. 2009;25(4):279–87.
Wasson K, Tate H, Hayes C. Food refusal and dysphagia in older people with dementia: ethical and practical issues. Int J Palliat Nurs. 2001;7(10):465–71. https://doi.org/ 10.12968/ijpn.2001.7.10.9902.
Xia W, Zheng C, Lei Q, Tang Z, Hua Q, Zhang Y, Zhu S. Treatment of post-stroke dysphagia by vitalstim therapy coupled with conventional swallowing training. J Huazhong Univ Sci Technolog Med Sci. 2011;31(1): 73–6. https://doi.org/10.1007/s11596-011-0153-5.