Частота
Рак щитовидной железы является наиболее распространенным эндокринным раком. Существует целый спектр биологического поведения, от индолентных, хорошо дифференцированных опухолей до чрезвычайно агрессивных, слабо дифференцированных или анапластических видов рака. [1,2] Рак щитовидной железы является наиболее быстро растущим злокачественным новообразованием в Соединенных Штатах как для мужчин, так и для женщин. С 1980 по 2006 год ежегодная заболеваемость раком щитовидной железы в США выросла с 4,33 до 11,03 случаев на 100 000 населения. Эта заболеваемость увеличилась к 2009 году до 14,3 на 100 000 человек [3]. Это почти трехкратное увеличение заболеваемости. Уровень заболеваемости с учетом гендерных факторов вырос с 6,5 до 21,4 = 14,9 на 100 000 женщин и почти в 4 раза выше, чем среди мужчин, с 3,1 до 6,9 = 3,8 на 100 000 мужчин. [3] Такое увеличение заболеваемости объясняется улучшением выявляемости небольших опухолей, в основном папиллярного рака щитовидной железы, с помощью УЗИ высокого разрешения [4]. Изменение объясняется, главным образом, увеличением заболеваемости папиллярным раком щитовидной железы (PTC). [5] В опубликованном отчете по результатам популяционного исследования говорится, что быстрый прирост заболеваемости раком щитовидной железы связан с оккультным раком, выявляемым визуалиционными методами диагностики шеи, со стабильным клинически обнаруженным раком щитовидной железы и смертностью от болезни [6]. Несмотря на это улучшенную детекцию, летальность остается неизменной на уровне 0,5 на 100 000 человек популяции. [7,8] Таким образом, рак щитовидной железы представляет собой уникальную задачу для лечащего врача по управлению ожиданиями пациентов, минимизации потенциально прижизненных осложнений, использованию соответствующего наблюдения наблюдения и выявлению пациентов с более агрессивными, плохо дифференцированными типами.
Классификация
Наиболее распространенным типом рака щитовидной железы является PTC, который составляет 80% всех случаев. Следующим по распространенным типом является фолликулярный рак щитовидной железы (FTC), который составляет 10-20% всех инцидентов. Вместе папиллярный и фолликулярный рак объединены понятием дифференцированный рак щитовидной железы (DTC), и оба они возникают из фолликулярных клеток щитовидной железы. MTC происходит из парафолликулярных C-клеток. На долю этой нейроэндокринной опухоли щитовидной железы приходится 5-10% всех случаев рака щитовидной железы, он встречается в семейных и спорадических формах. Наконец, анапластический рак щитовидной железы (ATC) является одним из наиболее агрессивных и высоко летальных типов рака. Он может развиваться из DTC, который дедифференцируется со временем, и также возникает de novo. [1,2,9,10] В первой части этого раздела о раке щитовидной железы обсуждается DTC, а во второй части рассматривается MTC (таблица 68.1, рис. 68,1).
Этиология
Экспозиция шеи внешнему облучению является одной из наиболее известных причин рака щитовидной железы. Этот риск связан с дозой облучения и возрастом и сохраняется в течение всей жизни. Облучение может быть вызвано внешним источником, используемым в диагностических и терапевтических целях, или внутренним излучением от пищи или жидкости, обладающей радиоактивностью. Ранее пациенты получали лучевую терапию в качестве лечения увеличенных миндалин или угрей на лице. Сегодня больные раком, например, болезнью Ходжкина, по-прежнему могут получать лучевую терапию. Кроме того, дети, подвергшиеся радиоактивному заражению в результате аварии на Чернобыльской АЭС, продемонстрировали повышенный уровень заболеваемости раком щитовидной железы. [11,12] На основании данных, полученных от пациентов с болезнью Ходжкина, дозы 40 Гр являются потенциально канцерогенными [13]. Эпидемиологические исследования показали, что от 7 до 9% пациентов, получивших от 5 до 10 Гр внешнего облучения, заболевают раком щитовидной железы. [14] Между экспозицией и диагностикой рака щитовидной железы обычно существует задержка в 10-20 лет, хотя сообщалось о гораздо более коротких периодах (таблица 68.2). [14]
Таблица 68.1. Гистологическая классификация рака щитовидной железы и его частота
| Гистология опухоли | Частота (%) |
| Дифференцированные карциномы | 81–87 |
| Папиллярная | |
| Фолликулярный вариант папиллярной | |
| Фолликулярная и клеток Гюртле | |
| Медуллярная | 6–8 |
| Анапластическая | 5 |
| Лимфома | 1–5 |
| Метастатическая | <1 |
В средовую этиологию рака щитовидной железы включено содержание йода в пище. Более высокая заболеваемость PTC наблюдается в регионах с высоким содержанием йода в рационе питания, таких как Тихоокеанское кольцо и Исландия. [15] В странах с дефицитом йода, напротив, отмечается более высокая заболеваемость FTC в дополнение к доброкачественным зобам щитовидной железы. Эти исследования противоречат многим факторам, которые связывают изменения в показателях DTC с потреблением йода. Этническая принадлежность, селен, зоб и канцерогены, вероятно, играют причинную роль. [16]
Рост количества исследований молекулярных маркеров, которые могут отличать рак от доброкачественных узлов, вело к лучшему пониманию генетических изменений в раке щитовидной железы. Например, 70% раковых заболеваний, обнаруженных у выживших в Чернобыле, имели перестройку RET и PTC генов (RET/PTC). Слияние домена, кодирующего тирозинкиназу RET протеина, с гетерологичной группой генов происходит в 20-40% случаев PTC и называется RET/PTC перестройкой. [17] Она часто встречаются в небольших многофокальных PTC, сопровождающихся воспалительным инфильтратом, часто наблюдаемым у людей, подвергающихся воздействию ионизирующего излучения, и у детей [18]. BRAF является членом сигнального каскада RAF-MEK-ERK серин/треониновых киназ, и мутация BRAF обнаруживается в 40–60% случаев PTC. [19,20] Точечная мутация V600E BRAF или мутации в другом члене этого сигнального пути, RAS, часто наблюдаются в случаях плохо дифференцированных PTC или ATC. [21] Анализ 27 PTC показал, что 70% несут мутантные гены, распределенные среди BRAF (60%), PIK3A (11%), TP53 (7%) и NRAS (4%). В том же исследовании распространенность мутантных генов в фолликулярной карциноме составила NRAS — 25%; KRAS — 0,5%; HRAS — 0,2%; TSHR — 11%; TP53 — 11%; и PTEN — 0,2%. [20] Большинство BRAF замен поддерживают протеин в каталитически активной форме, что приводит к конститутивной активации сигнального каскада RAF-MEK-ERK и постоянной митогенной активности. По данным многочисленных исследований была оценена очень высокая специфичность, равная приблизительно 99% BRAF V600E, с очень низкой чувствительностью для исключения злокачественности. [22]
Более высокая распространенность мутаций BRAF V600E наблюдается в папиллярной микрокарциноме щитовидной железы с метастазами в лимфатические узлы и рецидивирующих опухолях [23–25].
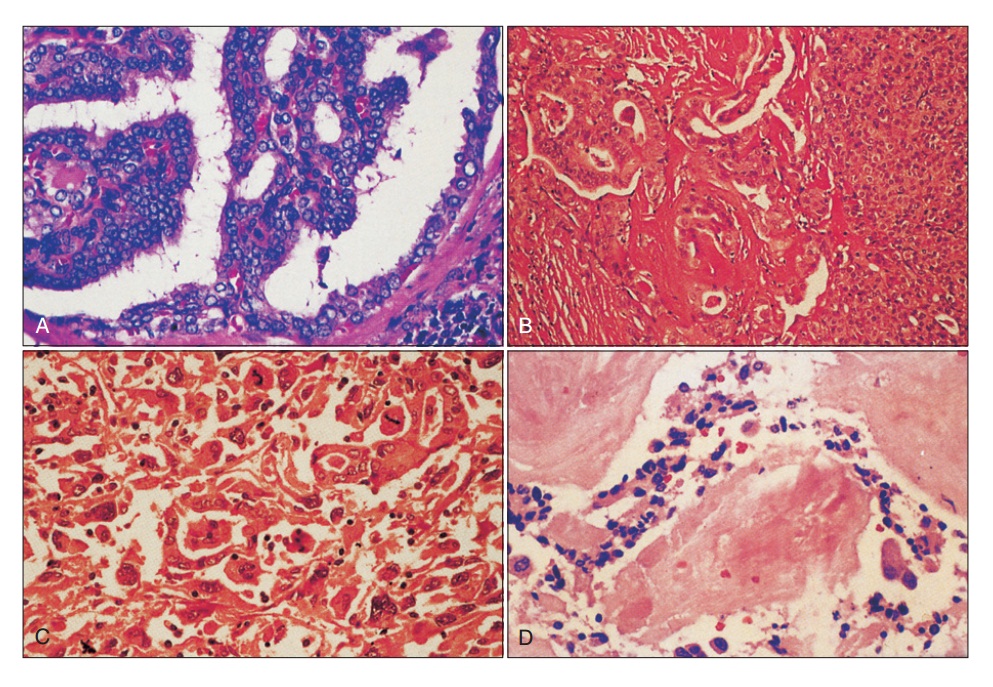

Фигура 68.1. Гистологические паттерны рака щитовидной железы. (A) Папиллярная карцинома. (B) Чистая фолликулярная карцинома. (C) Анапластическая карцинома. (D) Медуллярная карцинома.
Ras протеины представляют собой гуанозин-трифосфатазы плазматической мембраны, активируемые рецепторами факторов роста. Мутации, которые результируют в их конститутивную активацию, ведут к онкогенезу. RAS мутации наблюдаются примерно в 40% случаев фолликулярного рака и в небольшой части PTC, особенно в фолликулярном варианте PTC. [26,27] Подобно RET/PTC перестройке, другая межхромосомная транслокация встречается в FTC. Промоторный элемент гена, кодирующего PAX8 (paired box 8), сливается с последовательностью, кодирующей PPARγ (peroxisome proliferator-activated receptor γ) ген, в 35% FTC. [21,28,29] Функциональные последствия PAX8/PPARγ перестройки остается неясными. RAS мутации также широко распространены в FTC, но, возможно, перестройки RAS и PAX8/PPARγ могут быть взаимоисключающими, позволяя предположить, что это два различных молекулярных пути развития FTC [21].
Для диагностической точности молекулярных маркеров, особенно для неопределенных узлов щитовидной железы, были расширены несколько панелей с включением мутационных/транслокационных точечных мутаций BRAF, NRAS, HRAS и KRAS, в дополнение к RET/PTC1 и RET/PTC3, с или без PAX8/PPARγ перестройкек [30–34] Мутации TERT промотора выявлены в 2–10% спорадических PTC [35]. Они могут сосуществовать с V600E мутацией BRAF в PTC и быть более агрессивными. [36]
Наряду с приобретенными поражениями генов, часть форм DTC также наследуются. DTC наблюдается в семейных синдромах, таких как синдром Гарднера (Gardner), синдром Коудена (Cowden) и синдром Вернера (Werner).[37,38] Семейный немедуллярный рак щитовидной железы (FNMTC), при котором у двух или более родственников первой степени диагностируется DTC в отсутствии другого синдрома, показывает аутосомно-доминантное поведение с неполной пенетрантностью и вариабельной экспрессией.[38–40] Анализ связи выявил несколько кандидатных генов для FNMTC, включая TCO1, MNG1, fPTC/PRN и NMTC1, но один ответственный ген не был идентифицирован. [41–44] Фактические данные показали, что от 5% до 10% DTC имеют семейную наследственность. [5] По сравнению со спорадическим DTC, FNMTC более агрессивен с повышенным рецидивом и сниженной безрецидивной выживаемостью, локальной инвазией, мультицентричностью, метастазами в лимфатические узлы, инвазией в окружающие структуры и комбинацией с хроническим лимфоцитарным тиреоидитом. [45,46] Однако в отсутствие пригодного генетического теста семейный скрининг нельзя провести, и FNMTC трудно отличать от спородических DTC. [47]
Классификация и прогноз
DTC широко делятся на папиллярные или фолликулярные. Фолликулярный вариант PTC имеет признаки как PTC, так и FTC, но классифицируется как подтип PTC (таблица 68.1 и рис. 68.1A). В целом, хорошо дифференцированный PTC имеет отличный прогноз с 5-летней выживаемостью более 97%. [7] Небольшие опухоли имеют лучший прогноз, чем большие. PTC размером менее 1 см называются папиллярными микрокарциномами, и о них сообщалось в 10–30% аутопсийных изучений. [48–50] В прошлом эти опухоли случайно находили в тиреоидэктомических образцах, но теперь они выявляются с возрастающей частотой УЗИ высокого разрешения. Считается, что у них отличный прогноз: частота рецидивов структурного заболевания составляет 1-2% в унифокальных PTC и 4-6% в мультифокальных PTC. [51,52] Однако некоторые из них могут вести себя более агрессивно, чем предполагалось ранее, и их менеджмент остается спорным. [53,54]
Возраст является другим важным фактором, определяющим прогноз DTC. Пожилые пациенты, как правило, имеют менее дифференцированные, агрессивные варианты и реже отвечают на радиоактивный йод (RAI). В этих случаях смерть наступает в результате локальной инвазии и обширных метастазов. Поэтому полнота резекции и внетиреоидное распространение являются двумя прогностическими показателями, используемыми во многих системах стадирования DTC. [55,56] Роль метастазов в лимфатические узлы в детерминировании DTC-специфической выживаемости остается спорной. Вовлечение лимфатических узлов является типичным для PTC, но точная частота метастазов в лимфоузлы зависит от метода их определения. Пальпируемые массы в лимфатических узлах наблюдаются у 5-10% пациентов с PTC, но УЗИ выявляет патологически положительные лимфатические узлы у 30% пациентов. [57,58]
Ultrasound features that are associated with thyroid cancer include nodule hypoechogenicity compared with the surrounding structures, microcalcifi irregular margins, and taller-than-wide shape as measured on a transverse view.[5] Only 2% of patients with FTC have lymph node metastases because the route of spread is mostly hematogenous, but treatment guidelines and retrospective studies frequently consider PTC and FTC together. Routine histologic examination of lymph nodes reveals DTC in 20% to 50% of patients (particularly papillary carcinoma), but when more detailed inspection is performed, up to 90% of patients with DTC will have lymph nodes with microscopic disease, smaller than 2 mm.[59,60] An increasing amount of high-quality evidence supports sonographic survey of cervical lymph nodes in all patients with thyroid nodules.[5] Historically, lymph node involvement was believed to increase local recurrence without affecting survival, and therefore surgeons took a conservative approach to lymph node dissection for DTC. Wada and colleagues demonstrated that patients with pathologically positive lymph nodes had a recurrence rate of 16.3% compared with 0% in patients without pathologically positive lymph nodes.[61] Whether metastatic lymph nodes are evident preoperatively appears to be an important factor determining recurrence. For example, Ito and colleagues found that if metastatic lymph nodes were not seen preoperatively, then the risk of nodal recurrence was only 1.5%. Of note, in this study of 590 patients with microcarcinomas, 40% of patients had lateral neck lymph node metastases identifi histologically after prophylactic neck dissection.[62] Hence, lymph node metastases do affect recurrence, and clinically apparent nodes are more important than pathologically positive nodes. The impact of lymph nodes on survival is less clear. Large series and population-based studies have suggested that there is a small but significant effect on survival.[63,64]
Таблица 68.2. Факторы риска злокачественности в нодулярной щитовидной железе
| Низкий риск ↔ Высокий риск | |||||
| Фактор | 1 | 2 | 3 | 4 | 5 |
| Возраст | |||||
| Престарелые | х | ||||
| Дети | х | ||||
| Пол | |||||
| Мужчина | х | ||||
| Женщина | х | ||||
| Низкие дозы облучения в детстве | х | ||||
| Семейная история | х | ||||
| Кистозная опухоль | х | ||||
| Солидная опухоль | х | ||||
| Несколько опухолей | х | ||||
| Одиночная опухоль | х | ||||
| Растущая опухоль | х | ||||
| Стабильная опухоль | х | ||||
| «Горячий» скан | х | ||||
| «Холодный» скан | х | ||||
| «Теплый» скан | х | ||||
| Тонкоигольная аспирация (-) | х | ||||
| Тонкоигольная аспирация (+) | х | ||||
| Ассоциированная цервикальная аденопатия | х | ||||
| Полное разрешение в ответ на тиреоидную супрессию | х | ||||
| Частичное разрешение в ответ на тиреоидную супрессию | х | ||||
| Нет ответа на супрессию | х | ||||
Таблица 68.3. TNM классификация злокачественных опухолей щитовидной железы
| Первичная опухоль (Т стадия) | |
| Tx | Опухоль не может быть оценена |
| T0 | Нет клинических признаков опухоли |
| T1 | Опухоль ≤2 см, ограниченная щитовидной железой |
| T2 | Опухоль >2 см и <4 см, ограниченная щитовидной железой |
| T3 | Опухоль ≥4 см, ограниченная щитовидной железой, или любая опухоль с минимальным внетиреоидным распространением |
| T4a | Опухоль любого размера, выходящая за пределы капсулы щитовидной железы и инвазирующая подкожные мягкие ткани, гортань, трахею, пищевод или возвратный гортанный нерв |
| T4b | Опухоль, которая инвазирует превертебральную фасцию или охватывает сонную артерию или сосуды средостения |
| Анапластические карциномы a | |
| T4a | Интратиреоидальные — хирургически резецируемые |
| T4b | Экстратиреоидальные — хирургически резецируемые |
| Регионарные лимфатические узлы (N стадия) | |
| Nx | Региональные узлы не могут быть оценены |
| N0 | Нет пальпируемых узлов |
| N1 | Метастазы в региональные лимфоузлы |
| N1a | Метастазы в лимфоузлы VI уровня (предтрахеальные, паратрахеальные, преларингеальные) |
| N1b | Метастазы в унилатеральные, билатеральные или контралатеральные шейные или верхние средостенные узлы |
| Отдаленные метастазы (М стадия) | |
| Mx | Метастазы не могут быть оценены |
| M0 | Нет признаков отдаленных метастазов |
| M1 | Имеются отдаленные метастазы |
a Все анапластические карциномы рассматриваются как Т4 опухоли.
From the Surveillance, Epidemiology, and End Results (SEER) database, comprehensive analysis showed that patients younger than 45 years with lymph node metastasis had small but significant risk of death compared with younger patients with no lymph node involvement.[65] Because of the questionable effect on mortality, lymph node status is not included in all of the staging systems available for DTC. For example, the AGES system considers age, grade, extrathyroidal extension, and size.[66] The AMES system uses age, distant (non–lymph node) metastases, extent of primary tumor, and size.[67] Some, such as the MACIS system (metastases, age, complete excision, invasion, and size), also account for the adequacy of surgical treatment.[68] Alternatively, staging systems developed by the Ohio State University,[69] the European Organisation for Research and Treatment of Cancer (EORTC),[70] the National Thyroid Cancer Treatment Cooperative Study (NTCTCS),[71] and the American Joint Committee on Cancer (AJCC)[72] all do consider lymph node status. The AJCC staging system is the most widely used (Таблица 68.3). It is also known as the TNM system because it considers tumor size (T), lymph node metastases (N), and distant metastases (M). Like many of the other thyroid cancer staging systems, it also considers age, with two different classifications for those younger and older than 45 years. In those younger than 45 years, patients with lymph node metastases are classified as stage I unless they have distant metastases (stage II) (Таблица 68.4).[72]
Таблица 68.4. Стадирование рака щитовидной железы
| Стадия | TNM |
| Пациенты моложе 45 лет | |
| I | Любой T, любой N, M0 |
| II | Любой T, любой N, M1 |
| Пациенты в возрасте 45 лет и старше | |
| I | T1, N0, M0 |
| II | T2, N0, M0 |
| III | T3, N0, M0 |
| T1–3, N1a, M0 | |
| IVA | T4a, N0–1a, M0 |
| T1–4a, N1b, M0 | |
| IVB | T4b, любой N, M0 |
| IVC | Любой T, любой N, M1 |
| Медуллярный рак щитовидной железы | |
| I | T1, N0, M0 |
| II | T2–3, N0, M0 |
| III | T1–3, N1a, M0 |
| IVA | T4a, N0–1a, M0 |
| T1–4a, N1b, M0 | |
| IVB | T4b, любой N, M0 |
| IVC | Любой T, любой N, M1 |
| Анапластический рак | |
| IVA | T4a, любой N, M0 |
| IVB | T4b, любой N, M0 |
| IVC | Любой T, любой N, M1 |
It is worth noting some important ways in which FTC differs from PTC (see Таблица 68.1, Fig. 68.1B). Pure follicular carcinoma tends to occur more in older patients, and it carries a worse prognosis than PTC. Follicular carcinoma is more common in women than in men by approximately three times.[73] Even when the disease is confined to the thyroid, 5% to 15% of patients ultimately die from the disease, although survival still extends decades as in PTC.[74] In addition to the prognostic factors common to the aforementioned DTC staging systems, prognosis in FTC depends on the degree of capsular and vascular invasion. Minimally invasive tumors are grossly contained within the thyroid but have microscopic foci of invasion into the capsule. Invasive tumors carry a worse prognosis and invade the capsule and vessels.[75,76] Hьrthle cell tumors of the thyroid are often classifi with follicular cancer because they are derived from the follicular cell. Both adenomas and carcinomas of the Hьrthle cell can occur, and differentiating them by cytologic assessment is difficult, as it is with follicular lesions.[77,78] Capsular and vascular invasion distinguish carcinoma from adenomas. Large Hьrthle cell cancers (>2 cm) have a higher recurrence rate, ranging from 21% to 59%.[77,79] Furthermore, Hьrthle cell cancers do not always concentrate iodine. For these reasons, Hьrthle cell carcinoma carries a worse prognosis compared with DTC. Adenomas have an excellent prognosis after resection and less than 2.5% demonstrate malignant behavior, but resection is recommended for larger adenomas because size is a major predictor of malignancy.[79,80]
As follicular or papillary cancers progress or dedifferentiate, their prognosis becomes much worse. Anaplastic cancers are at the least differentiated end of the spectrum and represent one of the most aggressive cancers, with 5-year disease-free survival and cause-specific survival rates of 0%.[81] ATC can arise from well-differentiated tumors, or it can also develop de novo (see Fig. 68.1C).[81] A group of tumors falls between well-differentiated thyroid cancers and anaplastic cancers. These cancers, called poorly differentiated thyroid cancers, are intermediate in terms of their histologic appearance and their biologic behavior.[1] Although the literature remains inconsistent about what constitutes poorly differentiated cancer, the best definition comes from Burman and colleagues: “poorly differentiated thyroid carcinoma is a concept proposed to include carcinomas of follicular thyroid epithelium that retain sufficient differentiation to produce scattered small follicular structures and some thyroglobulin (Tg), but generally lack the usual morphologic characteristics of papillary and follicular carcinoma.”[82] These tumors include insular, large cell, tall cell, columnar cell, solid, and diffuse sclerosing variants.[1,82] In patients with these variants, the cancer tends to recur and metastasize. Furthermore, dedifferentiation of thyroid cancers leads to underexpression or disordered assembly of the sodium-iodide symporter, decreasing the usefulness of RAI for treating micrometastatic disease or detection of metastases.[83] For these reasons, poorly differentiated thyroid cancers have a 51% disease-free survival rate and a 70% cause-specific survival rate at 5 years.[1,81]
Primary lymphoma of the thyroid is not as common as DTC. Older women or patients with Hashimoto thyroiditis are at highest risk for developing thyroid lymphoma.[84] These tumors typically manifest as a rapidly expanding mass causing pain and compressive symptoms. Flow cytometry of cytologic specimens can sometimes be used to make the diagnosis, but the condition might be mistaken for Hashimoto thyroiditis. Consequently, a core biopsy is sometimes necessary when this diagnosis is suspected. Most are B-cell lymphomas treated with chemotherapy and radiation. Surgery is occasionally needed for palliation.[84] Prognosis depends on the histologic subtype.
Диагноз
Just as with a newly discovered mass anywhere else in the body, the workup of a thyroid nodule begins with a thorough history and physical examination. A strong family history of thyroid cancer or cancer syndromes or a history of radiation exposure to the head and neck or total-body radiation for bone marrow transplantation [85] should raise the suspicion of thyroid cancer. Rapid growth and/or hoarseness with compressive symptoms may indicate that the thyroid nodule is thyroid lymphoma or a poorly differentiated thyroid cancer.[1,2,14] On examination, malignant nodules are harder and fixed, whereas a nodule that is rubbery or soft and moves easily with deglutition is reassuring but not diagnostic of a benign nodule. Nonpalpable nodules have the same risk of malignancy as palpable nodules of the same size.[86–89] Cervical lymphadenopathy also increases the likelihood that a thyroid nodule is malignant.[2,90]
Лабораторные исследования
Because the management of patients with functional thyroid nodules differs from that of patients with nonfunctional nodules, obtaining a thyroid-stimulating hormone (TSH) measurement early in the workup of a thyroid nodule can efficiently identify patients with a nodule and hyperthyroidism. In this subset of patients with a suppressed TSH, an iodine-123 (123I) scan can distinguish a solitary toxic nodule from a toxic multinodular goiter and Graves disease. A solitary hyperfunctioning nodule is rarely malignant, and fi e-needle aspiration (FNA) biopsy or further cancer workup is rarely necessary. The one exception is that functioning nodules in children do carry a higher risk of malignancy.[91] If thyroid radionuclide scanning is undertaken, “cold” nodules should undergo FNA biopsy because 10% to 20% of cold nodules are malignant.[92,93] A higher serum TSH is an independent risk factor for predicting malignancy and is associated with more advance disease.[94,95] Other laboratory tests can be helpful once the diagnosis of a certain type of thyroid cancer has been made. For example, measuring serum Tg in patients with DTC can assist with the long-term follow-up of patients treated for DTC.[96–98] Although Tg levels can be elevated in patients with DTC, the test is insensitive and nonspecifi for diagnosing cancer, elevations in Tg can occur in benign thyroid disorders, and the American Thyroid Association (ATA) guidelines do not recommend routine preoperative Tg measurement for patients with DTC. After a total thyroidectomy, however, elevations in Tg can reliably indicate recurrent or metastatic disease.[5] Different threshold Tg levels can indicate recurrence depending on the concomitant TSH level. It should be emphasized, however, that there is no role for Tg measurement in the initial evaluation of thyroid nodules.
Measurement of serum anti–thyroid peroxidase (TPO) antibodies may be helpful in patients with high TSH levels suggestive of autoimmune thyroiditis; however, routine measurement is not necessary.
Тонкоигольная аспирационная биопсия
FNA biopsy remains the gold standard for evaluating thyroid nodules. Most clinical practice guidelines recommend FNA biopsy for nodules greater than 1 cm in largest dimension with a high to intermediate sonographic pattern.[5,92,99] Biopsy can be performed on nodules larger than 1.5 cm with few suspicious characteristics. For nodules larger than 1 cm with suspicious sonographic features of extrathyroidal extension or associated lymphadenopathy, FNA should be performed in selected patients such as those with family history of thyroid cancer.[100] When the FNA result is clearly benign or malignant, then the decision for further treatment, including thyroidectomy, becomes evident. The false-negative rate for FNA biopsy is 1% to 3% (Таблица 68.5). The false-negative rate increases to 10% to 15% when the nodule is large (>4 cm).[99,101] Other clinical scenarios in which the clinician should not always trust a benign FNA result include patients with a family history of thyroid cancer, patients with a history of radiation exposure, and cystic nodules.[102] Ultrasound guidance can improve the accuracy of FNA biopsy by confi that the nodule is actually being sampled and by enabling targeting of the most suspicious portions of the nodule (e.g., the wall of a cyst). This is especially true for nondiagnostic cytologic findings (>25%–50% cystic component) or nonpalpable or posteriorly located nodules.[103,104]
Nodules that do not meet FNA criteria, such as suspicious subcentimeter nodules in individuals older than 60 years, single nodules with well-defined margins, and nodules with a rim of normal thyroid parenchyma that is larger than 2 mm, can be observed.[100]
FNA results are classified according to the Bethesda criteria, which indicate the risk of malignancy (see Таблица 68.5). One of the limitations of cytologic evaluation of thyroid nodules is that it cannot distinguish between adenoma and carcinoma in follicular lesions.[92,101] Therefore lobectomy with permanent histology may be the best way to make a defi e diagnosis in follicular or indeterminate lesions. Furthermore, 20% to 30% of FNA results fall into the category of indeterminate cytologic findings, such as atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS).[105] Currently, many centers have turned to molecular analysis of FNA specimens to help distinguish follicular lesions. Cytologic specimens are analyzed for a panel of mutations, including BRAF, RAS, RET/PTC, and PAX8PPARγ rearrangements. The seven-gene mutational panel, most useful when surgery is favored, has sensitivity that ranges from 44% to 100%.[32–34] Although this is an exciting area of research, the clinical usefulness of the various gene panels has varied, and more prospective data are needed.[19,21,28,81,106]
Not only can ultrasonography improve the accuracy of FNA biopsy, but it is also an important tool in evaluation of thyroid nodules because it is used to measure the size and features of the nodule and it can also reveal additional nonpalpable nodules. Ultrasound examination alone can increase the clinician’s suspicion for malignancy if the nodule has fine microcalcifications, irregular borders, chaotic vascular patterns or peripheral vascularity, cystic aspect, or hyperechogenicity.[109] In addition, ultrasound scanning can be used to evaluate the lymph nodes in both the central and the lateral neck compartments, which may prompt additional FNA biopsy of suspicious lymph nodes or result in alterations in the surgical plan. Although ultrasound examination is highly operator dependent, it is noninvasive and does not involve any radiation or contrast agent risk to the patient. Highresolution ultrasonography can also demonstrate extracapsular invasion and subtle lymph node involvement.[104,110–115] Consequently, ultrasonography is the preferred method to evaluate the thyroid and cervical lymph nodes. Although routine screening is not recommended for all patients, those with a strong family history or radiation exposure can undergo ultrasound screening for thyroid nodules. Use of positron emission tomography (PET) and/or computed tomography (CT) scan is helpful for identifying lung or bone tumors in patients at risk for metastases.[112,116]
Highly aggressive cancers that may invade local structures, extend into the chest, or demonstrate poorly differentiated cytologic features require careful preoperative planning. In such cases, CT becomes a helpful preoperative imaging study in planning en bloc resection of other organs aside from the thyroid, understanding the extent of vascular involvement, determining if a thoracic incision is necessary, and planning for reconstruction.[1,112,116,117]
Chest CT or PET-CT imaging studies performed for other medical indications often reveal thyroid nodules incidentally. PET scans demonstrate thyroid masses during the workup and staging of other cancers. This subset of incidentally discovered thyroid nodules deserves special attention because up to 50% of fl odeoxyglucose (FDG)-avid thyroid nodules will contain thyroid cancer. Therefore, PET-positive thyroid nodules should undergo FNA biopsy.
Таблица 68.5. Система Bethesda для цитопатологии щитовидной железы
| Категория новообразования | Риск злокачественности (%) | Рекомендуемый менеджмент |
| Недиагностированное или недостаточное для диагноза | 1–4 | Повторная FNA под ультразвуковым контролем |
| Доброкачественное | 0–3 | Клиническое наблюдение |
| Атипия неопределенного значения (AUS) или фолликулярное поражение неопределенного значения (FLUS) | 5–15 | Повторная FNA a |
| Фолликулярное новообразование или подозрительное на фолликулярное новообразование | 15–30 | Лобэктомия |
| Подозрительное на злокачественное | 60–75 | Лобэктомия с или без замороженной секции или полная тиреоидэктомия |
| Злокачественное | 97–99 | Полная тиреоидэктомия |
a Лобэктомия также может рассматриваться в зависимости от клинических или сонографических характеристик. FNA, Fine-needle aspiration.
Лечение
В лечении DTC участвуют хирург, эндокринолог, специалист по радиационной медицине, а иногда и радиолог-онколог. Междисциплинарный подход лучше всего подходит для пациентов с DTC.
Хирургия
The extent of surgery for DTC remains controversial. This is especially true for small, encapsulated, well-differentiated tumors, and tumors smaller than 1 cm (microcarcinomas). These are discussed further later, but for most DTCs 4 cm or larger that were diagnosed preoperatively, most clinicians recommend a total thyroidectomy.[5] Controversy still exists for lesions larger than 1 cm and smaller than 4 cm with lowor intermediate-risk features. Contrary to the previous guideline recommendations,[118] the new guidelines [5] recommend either total thyroidectomy or lobectomy for treatment. Thyroid lobectomy may be sufficient for this type of lesion without extrathyroidal extension and with no evidence of lymph node metastasis. This is based on evidence from properly selected patients that showed similar clinical outcomes with either surgical plan.[119–123] Furthermore, lobectomy can obviate the need for exogenous thyroid hormone. Finally, because the current practice for follow-up depends more on ultrasound findings and Tg measurement than on whole-body RAI, total thyroidectomy is no longer needed to justify postoperative RAI.[5] Analysis of 5432 patients with PTC from the SEER database found no difference in 10-year overall survival between total thyroidectomy (4612 patients) and thyroid lobectomy (820 patients).[122] Results from two other single-center studies showed excellent survival in properly selected patients who underwent lobectomy.[119,123] Without evidence of high-risk features, initial lobectomy of 1to 4-cm thyroid carcinomas is more cost-effective after 3 years of follow-up.[124] On the other hand, the rationale for total thyroidectomy is based on tumor biology and current treatment modalities. DTC, especially PTC, tends to be multicentric, with up to 80% of patients having multiple tumor foci and 60% having bilateral disease when a thorough pathologic examination of the contralateral lobe was performed.[10,53,112] A study showed that 43% of patients who underwent thyroidectomy for 1to 4-cm thyroid cancers had high-risk characteristics that would have necessitated complete thyroidectomy if lobectomy had been used as initial treatment.[125] A total thyroidectomy as the initial procedure obviates the need for reoperative surgery to remove the contralateral lobe should a recurrence become detected. Second, experienced thyroid surgeons can safely perform a total thyroidectomy, with permanent complications such as recurrent laryngeal nerve injury and hypoparathyroidism occurring at a rate of less than 2%.[54,117] RAI therapy for ablation of microscopic disease becomes most effective when the thyroid remnant is small or absent. Tg measurement and radioiodine whole-body scanning are highly sensitive modalities for detection of recurrent or metastatic disease, but these two methods are most effective when no thyroid tissue remains in the neck.[2,126]
Most low-risk cancers carry an excellent prognosis regardless of the extent of thyroidectomy, and there are no randomized prospective trials comparing total thyroidectomy and thyroid lobectomy in this group of patients. In addition, radioiodine may have limited usefulness in low-risk patients.[10,53] For these reasons, some researchers favor thyroid lobectomy in low-risk patients. For example, Shaha and colleagues have reported 20-year follow-up findings in 465 patients with low-risk DTC. Although the lobectomy group had more local recurrence compared with the total thyroidectomy group (4% versus 1%), there was no statistical significance.[127] Similarly, other groups have also failed to demonstrate any signifi effect on survival.[128–130] In contrast, large retrospective series have demonstrated improvement in recurrence rates for total thyroidectomy compared with less extensive operations.[69,131–133] In a frequently cited study, Mazzaferri and colleagues reported on 1355 patients with a mean follow-up of 15.7 years. Patients treated with total thyroidectomy experienced signifi improvements in recurrence rate (26% versus 40%, P < .02) and mortality rate (6% versus 9%, P = .02) compared with less extensive resections.[134] Owing to concerns regarding the accuracy of risk stratification and complications in these retrospective studies, current guidelines recommend lobectomy for small (<4 cm), unifocal, well-differentiated tumors with no lymph node metastases or extrathyroidal extension.[5]
Another hotly debated topic related to the extent of initial surgery for DTC is the role of prophylactic central neck dissection. The most recent guidelines are consistent with the 2009 guidelines, which state that “prophylactic central neck dissection may be performed, especially in patients with advanced primary tumors (T3 or T4) or clinically involved lymph nodes” and “total thyroidectomy without prophylactic central neck dissection may be appropriate for small (T1 or T2), noninvasive, clinically node-negative patients.”[5,97]
The central neck lymph nodes are also classified as level VI lymph nodes and include the paratracheal, perithyroidal, and precricoid lymph nodes. These nodes are found along and behind the recurrent laryngeal node, and frequently surround the lower parathyroid gland. Although the level VI lymph nodes contain macroscopic disease in 10% of cases, when they are removed prophylactically, 32% to 69% of patients will have microscopic metastases.[135–137]
Proponents of prophylactic central neck dissection argue that the initial operation is the safest time to remove central neck lymph nodes to prevent local recurrences and the complications associated with reoperative surgery in the central neck. Wada and colleagues found the recurrence rate in patients treated with therapeutic lymph node dissection to be 21%, whereas patients who underwent prophylactic neck dissection experienced a recurrence rate of only 0.43%. Important to note, those patients without clinically overt nodal disease who did not undergo prophylactic central neck dissection also experienced a very low recurrence rate of 0.65%. Hence, the absolute differences in recurrence are minuscule.[61] Several other studies also have supported the concept that microscopically positive lymph nodes rarely progress to recurrence, especially after postoperative RAI ablation.[138–140] Clinically evident lymph node metastases place patients at higher risk for recurrence, and these patients clearly benefit from therapeutic lymph node dissection. Prophylactic central neck dissection reduces an already low recurrence rate and potentially eliminates or reduces the need for RAI but is also associated with risks such as hypoparathyroidism. The risk-to-benefit ratio may favor prophylactic central neck dissection in a subset of patients, but the putative risk factors that define such a subset remain unknown.[112,141,142]
Evidence supports a selective approach of prophylactic level VI node dissections in patients with clinically or radiographically involved lymph nodes or intraoperative detection of metastatic lymph nodes (cN1).[143–145] Thyroidectomy begins with proper patient positioning in the semirecumbent position with the neck extended (Fig. 68.2). A transverse, curvilinear incision is made in a suiТаблица skin crease at or beneath the level of the cricoid cartilage. Traditionally a Kocher collar incision was used, but this requires a very large dissection superiorly to reach the upper pole of the thyroid, placing the patient at risk for postoperative seroma. Intraoperative ultrasound guidance can help in assessing the upper extent of the gland and placing the incision appropriately. Superior and inferior subplatysmal flaps are raised to create a working space around the thyroid. The strap muscles are divided in the median raphe and are retracted laterally. It is rarely necessary to transect the strap muscles, but this can be accomplished if the tumor is large or adherent to the overlying muscles. The perithyroidal soft tissue is swept off the gland bluntly to identify the boundaries of the thyroid. Because the thyroid is most fixed at the upper pole, these vessels are divided first. Much of this can be accomplished by using energy devices such as the Harmonic scalpel or LigaSure, but in larger vessels clips and/or ties may be required. The upper pole vessels should be ligated close to the thyroid capsule to avoid injuring the external branch of the superior laryngeal nerve. In addition, the surgeon should remain vigilant for the upper parathyroid gland, which is frequently located near the upper pole vessels. Next, the thyroid gland is reflected medially. To accomplish this, the middle thyroid vein is ligated. In addition, dividing the thyroid isthmus can also facilitate medial rotation, assuming that the tumor is not located within the isthmus. Before any structures along the medial border of the gland are divided, the recurrent laryngeal nerve must be identified and its course dissected. The nerve is found medial to the upper parathyroid gland and lateral to the lower parathyroid. The parathyroid glands must also be identified and dissected free from the thyroid on an intact vascular pedicle. Once the recurrent laryngeal nerve has been identified, the branches of the inferior thyroid artery can be divided along the thyroid capsule. The inferior pole also is mobilized with a combination of blunt dissection and ligation of the inferior thyroid artery. The thyroid is then dissected off the anterior surface of the trachea with electrocautery or other energy devices to divide the small vessels contained within the ligament of Berry. Performing the identical procedure on the contralateral lobe completes a total thyroidectomy.
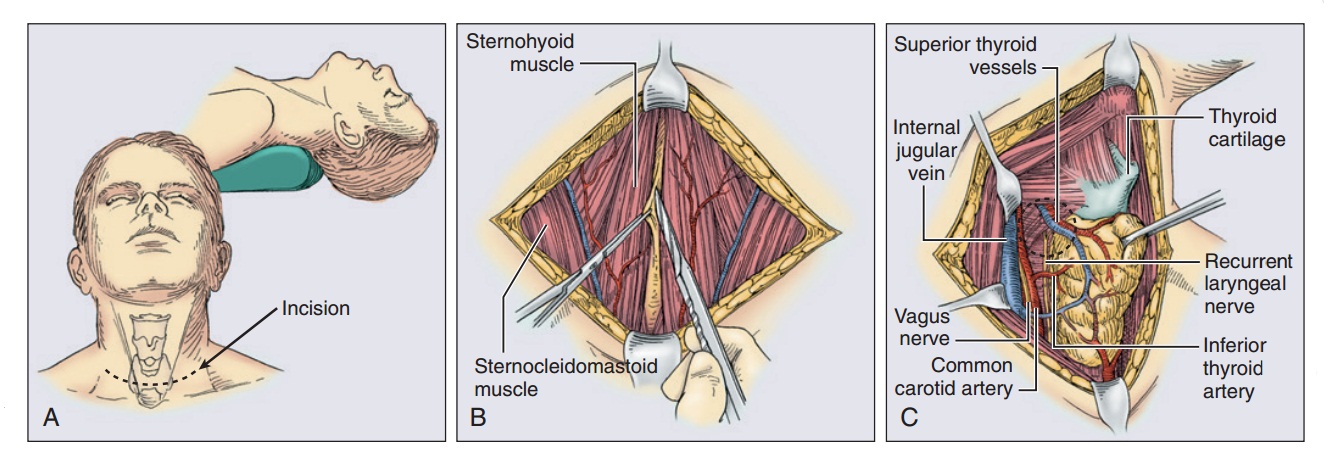
Фигура 68.2. Тиреоидэктомия. (A) Пациент находится в положении в разгибанием шеи. Щитовидная железа достигается через разрез Kocher collar, который обычно делается примерно на 2 см выше надгрудинной вырезки. (B) strap мышцы разделяются по средней линии для выделения щитовидной железы. (C) The strap muscles are retracted laterally and the thyroid is retracted medially, exposing the structures of the midneck. The recurrent laryngeal nerve can be seen lying within the tracheoesophageal groove. (D) The superior pole vessels are individually clamped and ligated as they enter the thyroid gland. Inferior thyroid vessels, in addition to the thyroidea ima vessels, are individually suture-ligated. (E) The dissection is completed by dissection of the thyroid gland off the trachea. The isthmus is then transected and can be oversewn with a suture for hemostasis.
Before passing the specimen off the fi the surgeon should examine it to make sure that there is no parathyroid tissue adherent to the gland. Any inadvertently removed parathyroid tissue can be finely minced and reimplanted into either the sternocleidomastoid or the strap muscles. Frozen section of a biopsy specimen of this tissue can be used to distinguish among fat, parathyroid, or lymph node; this will also help in avoiding autotransplanting cancer-bearing lymph nodes back into the patient. Two or three pockets are created within the muscle, and the minced parathyroid tissue is divided among these pockets. Each pocket should be marked with permanent suture so that it can easily be found in a reoperative setting.
Preoperative FNA or intraoperative frozen section can be used to confi that enlarged lymph nodes seen on ultrasound harbor metastatic disease. Cytologic or pathologic confi of lymph node metastases should prompt the surgeon to perform a compartment-oriented lymph node dissection. Lymph node sampling or “berry-picking” should be avoided, because this leaves behind lymph nodes that likely contain microscopic disease, which then become more difficult to excise in a reoperative setting.
Although “skip” metastases directly to the lateral compartment can occur in PTC, the central neck nodes (level VI) are usually the first nodes to receive drainage from the thyroid (Fig. 68.3). The boundaries of the central neck are the carotid sheathes laterally, the hyoid bone superiorly, and the innominate artery inferiorly.[146] Lymphadenectomy in this area requires skeletonizing the recurrent laryngeal nerve along its entire cervical course, and removing all the fibrofatty tissue along the trachea. Frequently, the lower parathyroid is invested in this tissue and becomes devascularized with this dissection.[141]
A lateral neck dissection usually involves dissection of levels II, III, and IV (see Fig. 68.3). This dissection puts the spinal accessory, phrenic, vagus, cervical sensory, sympathetic trunk, hypoglossal, greater auricular, and marginal mandibular branch of the facial nerves at risk. The extent of node dissection should be guided by preoperative and intraoperative ultrasound findings. Usually the great vessels can be preserved, but more aggressive tumors can invade the internal jugular vein, and it should be sacrificed in this scenario. In addition, to nerve injury, chyle leak is another complication of lateral neck dissection.[112,117]

Фигура 68.3. The central neck nodes (level VI) are usually the first nodes to receive drainage from the thyroid.

Фигура 68.4. (A) Whole-body scan acquired 24 hours after administration of 2 mCi of iodine-123 (123I). (B) Spot view of the neck and chest. There are several areas of uptake indicative of residual thyroid and functioning metastases in cervical lymph nodes. (C) Posttherapy scan made 7 days after administration of 150 mCi of 123I. There is intense uptake in the region, but the resolution is not as good as with 123I. There is faint uptake in the liver on the posttreatment scan as a result of metabolism of radioiodinated thyroid hormones at that site.
Радиоактивный йод
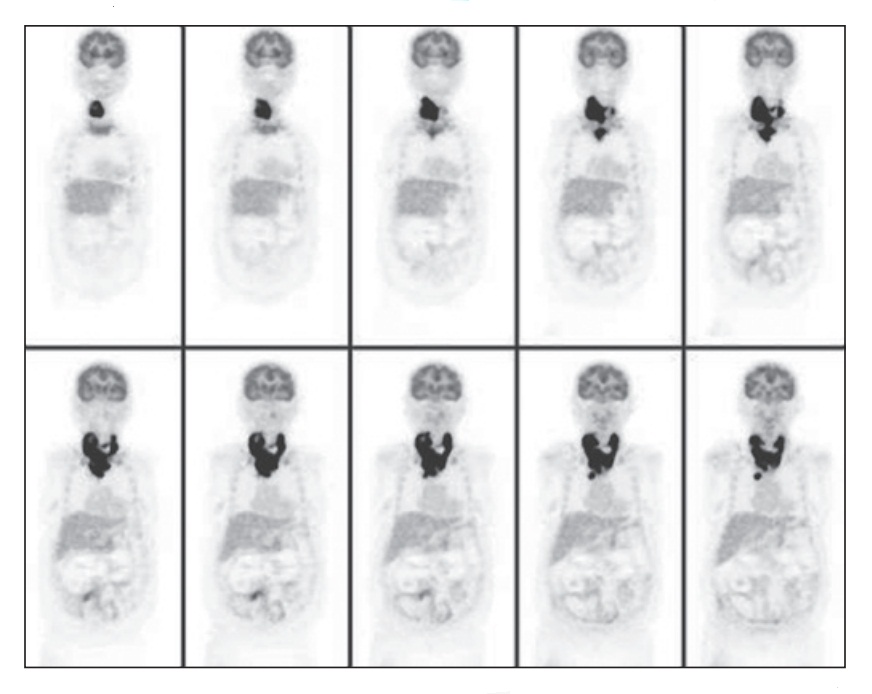
Remnant ablation with RAI is the standard adjuvant treatment for selected patients with DTC. Because the primary goal of RAI is remnant ablation, it can be administered only after a total or near-total thyroidectomy; otherwise the radioactive isotope will be absorbed by the remnant thyroid and will not destroy any micrometastatic disease as intended. In addition, RAI can be used as an adjuvant therapy after total thyroidectomy to improve disease-free survival or as a therapeutic modality to improve survival in patients with persistent disease.[5] RAI is administered 1 to 3 months postoperatively as iodine-131 (131I) as sodium iodide in an oral form whose half-life is 7 to 8 days (Figs. 68.4 and 68.5). Consensus guidelines recommend a dose of 30 to 100 mCi for patients with low-risk tumors and higher doses (100–200 mCi) for patients with residual disease, suspected microscopic disease, or more aggressive histologic subtypes (i.e., tall cell, columnar cell, or insular variants).[5,147–149] To stimulate intracellular uptake of the isotope, the TSH concentration should be at least as high as 30 mU/L. There are two methods for achieving such an elevation in TSH. The traditional method requires the patient to withdraw from thyroid hormone replacement over 4 to 6 weeks.[2,150] A newer method is to administer recombinant human thyroid-stimulating hormone (rhTSH). rhTSH is administered in the form of intramuscular injections on 2 consecutive days followed by RAI on the third day. The advantage of this method is that the patient does not experience an extended period of hypothyroidism as with hormone withdrawal. However, long-term data on the effectiveness of rhTSH compared with traditional withdrawal are lacking, although it appears effective for low-risk and intermediate-risk patients; there is no available evidence to support rhTSH use in patients with high risk of disease-related mortality and morbidity.[5] The US Food and Drug Administration (FDA) approved rhTSH for thyroid remnant ablation in patients who do not have evidence of metastatic disease.[151,152] In addition to increasing the TSH level, clinicians should also prepare patients by instructing them to follow a low-iodine diet for 1 to 2 weeks before RAI treatment. This diet requires patients to avoid foods that contain iodized salt, dairy products, eggs, seafood, soybeans or soy-containing products, and foods colored with red dye No. 3.[148,149]
Фигура 68.5. Positron emission tomography (PET) scan of a 75-year-old woman with anaplastic cancer of the thyroid. Images were acquired 1 hour after intravenous injection of 15 mCi fluorine-18 fluorodeoxyglucose (FDG). There is intense uptake of FDG in the undifferentiated cancer.
Although some studies have shown no benefit to RAI therapy,[153,154] other studies have demonstrated a reduction in locoregional recurrences and distant metastases.[69,131] As with the controversy over the extent of thyroidectomy, the benefit of RAI for low-risk patients remains unclear.[150] The most recent ATA (2016) guidelines recommend remnant ablation for all but the lowest-risk patients (unifocal, well-differentiated tumor, <1 cm in size, confined to the thyroid gland without lymph node metastases or multifocal papillary carcinoma with no other adverse fi 5 These recommendations are supported by multiple systematic reviews including one that was published in 2015.[155–157] In these low-risk patients, most of the available evidence suggests that RAI does not improve disease-specific or disease-free survival.[158–161] The National Comprehensive Cancer Network (NCCN) guidelines require a more thorough evaluation for the extent of remaining disease after thyroidectomy, with a radioiodine scan 1 to 12 weeks postoperatively. RAI ablation is not recommended if the stimulated Tg is less than 1 ng/mL and the radioiodine scan result is negative.[147]
Some studies have shown an increase in the risk of development of secondary malignancies after RAI therapy. This has been examined with use of the National Cancer Institute’s SEER database. Brown and colleagues found that patients treated for DTC had significantly higher rates of nonthyroid second primary malignancies than expected in the general population. Although the excess risk was relatively small, it was greater in the subset of patients who were treated with RAI.[162] Iyer and colleagues specifically examined low-risk patients (T1N0) treated with RAI and found that their excess absolute risk was 4.6 excess cases per 10,000 person-years at risk.[163] As discussed earlier, RAI clearly benefits patients with larger tumors and metastatic disease, but the increased risk of secondary malignancies in low-risk patients in whom the long-term benefit of RAI is questionable means that careful patient selection for RAI treatment is necessary.
Hematologic malignancies are the most common secondary malignancies after RAI, but there is also an association with kidney, breast, bladder, skin, and salivary gland cancers.[164–166] The more commonly noted side effects after radioiodine treatment include dry mouth, mouth pain, salivary gland swelling (sialadenitis), altered smell and taste, conjunctivitis, and fatigue. Women should not be pregnant at the time of treatment, nor should they become pregnant for at least 6 months after treatment. Similarly, men should avoid conception for at least 6 months after treatment.[149,156,166,167] Although a study has shown that BRAF V600E mutations signifi reduce sodium-iodine symporter expression and RAI uptake,[168] there is no clear role of molecular testing in guiding postoperative RAI.[5,169]
Супрессия тироксина
Because all cells of follicular origin depend on TSH for growth, TSH suppression through the administration of supraphysiologic doses of levothyroxine (T4) remains an important strategy for maintaining disease-free survival and overall survival.[170] For high-risk patients with incomplete resection, tumor invasion into adjacent structures, or distant metastases, the physician should initially titrate T4 dosage to a TSH level below 0.1 mU/L. Lower-risk patients should be treated to achieve a TSH level at or slightly below the lower limit of normal (0.1–0.5 mU/L).[5,147] Once patients remain disease-free for at least 2 years, their TSH suppression can be liberalized to within the reference range. Patients with persistent disease should be kept at a TSH level below 0.1 mU/L indefinitely. TSH suppression carries risks of arrhythmias, anxiety, and osteoporosis. The risks and benefits should be carefully considered, particularly in older patients. Because of the risk of bone loss, the NCCN guidelines recommend daily calcium and vitamin D supplementation for patients on TSH suppression.[147]
Облучение внешними лучами
Although 131I is the preferred adjuvant therapy for thyroid carcinoma, external beam radiation sometimes plays a role in treating this disease. Persistent, recurrent, anaplastic, or poorly differentiated tumors may fail to take up 131I. Treatment of anaplastic thyroid tumors almost always includes external beam radiation because these tumors often cannot be completely resected and do not concentrate iodine. Although no improvement in overall survival has ever been documented, external beam radiation is often given after resection of poorly differentiated tumors to reduce the risk of local relapse.[171] The group at Memorial Sloan Kettering Cancer Center has found that up to 85% of poorly differentiated tumors display some iodine avidity, and therefore treatment with RAI may remain worthwhile. Patients with incompletely resected tumors, unresecТаблица disease, and locoregional recurrence in a previously operated field may benefit from external beam radiation.[1,171,172]
Химиотерапия
Because RAI often can be effective treatment for well-differentiated tumors that have metastasized, cytotoxic chemotherapy has not been extensively evaluated for metastatic thyroid cancers. For large burdens of disease, anaplastic cancers, or poorly differentiated tumors that are not iodine avid, chemotherapy becomes an important treatment component after surgery or if the tumor is not resectable. In these situations, chemotherapy confers minimal effects because these tumors carry a very poor prognosis. Historically, doxorubicin was the most effective single agent. Combination therapy with doxorubicin and cisplatin resulted in modest objective response rates.[173,174] Newer, targeted therapies have shown some promise. Small-molecule tyrosine kinase inhibitors (such as sorafenib or sunitinib) and antibodies (anti–vascular endothelial growth factor [VEGF]) should be considered in the context of ongoing clinical trials.[147,175–177] Sorafenib and lenvatinib have been approved for use in the United States for patients with advanced RAI-refractory DTC. Although there was no improvement in overall survival with sorafenib and lenvatinib, both were associated with prolongation of progression-free survival (PFS) by 5 months.[178,179]
Рецидив рака
For most DTCs, the long-term survival rate exceeds 95%. Even though disease-specific mortality rates remain quite low (<1%), recurrence rates exceed mortality rates. For low-risk tumors, the locoregional recurrence rates range from 2% to 10% [180] and distant recurrence rates are 1% to 2%.[51,181] Higher-risk tumors (larger size, extrathyroidal extension, cervical lymph node metastases) carry recurrence rates of 21% to 68%.[62,180] For these reasons, a tailored approach to follow-up and treatment of recurrent disease best serves patients with DTC.
Наблюдение
The original tumor characteristics and operative findings dictate the follow-up schedule for DTC. Surveillance consists of measuring serum Tg, TSH, and anti-Tg antibodies in addition to imaging. Cervical ultrasound is a highly sensitive test for detection of metastatic lymphadenopathy. Elevations in Tg in the absence of cervical disease seen on ultrasound suggest distant metastases.[5,56] Whole-body radioiodine scanning is sensitive for detecting iodine-avid bone or pulmonary metastases.[96,99] Poorly differentiated, aggressive tumors will not concentrate iodine but can be detected with CT scan or FDG-PET (see Fig. 68.5).[83]
The frequency of surveillance depends on the original tumor characteristics and the AJCC stage.[72] For lower-risk tumors, physical examination, cervical ultrasound, and measurement of TSH, Tg, and anti-Tg antibodies should be performed every 12 months. These studies can be scheduled 3 to 6 months after the initial RAI treatment in patients with high-risk tumors.[5,147,177]
Лечение рецидивирующего канцера
Recurrence in the neck or cervical lymph nodes is best treated surgically. The decision-making process of surgical resection should be based on clinically apparent, macroscopic nodal disease confirmed with neck ultrasound or CT scanning, rather than depending on Tg elevation alone.[143,182,183] Furthermore, small nodal recurrence (<8 mm in the central neck or <10 mm in the lateral neck) can be managed with active surveillance.[182,184–186]
Other treatment options such as percutaneous ethanol injection (PEI) have shown some success in retrospective studies limited by small sample sizes.[187,188] External beam radiation should be considered for recurrent disease that is unresecТаблица or not iodine avid (Fig. 68.6). Percutaneous ultrasound-guided laser ablation has shown promising results for metastatic nodal disease.[189] RAI cannot ablate bulky nodal disease. After surgical resection, radioiodine can effectively ablate micrometastatic disease throughout the body.
Medical treatment for recurrent or metastatic disease consists of maintaining TSH suppression. Depending on the location of recurrence, health of the patient, tumor risk stratification, and patient preference, patients with metastatic disease can be referred for experimental protocols using targeted therapies, can undergo traditional cytotoxic chemotherapy, or can be treated with watchful waiting and supportive care.[177] A multidisciplinary team experienced with metastatic thyroid cancers best handles these decisions.
Медуллярный рак щитовидной железы
The prevalence of MTC has decreased from previously reported rates of 5% to 10% of all thyroid cancers to 1% to 2%. This is because of the increased incidence of PTC.[190] Unlike DTC, MTC arises from the parafollicular C cells instead of the follicular epithelium (see Fig. 68.1D). Hence, MTC is a neuroendocrine tumor (NET), and it shares some properties common among neuroendocrine cancers, including secretion of peptide hormones such as calcitonin, serotonin, or vasoactive intestinal peptide (VIP). Most cases of MTC are sporadic, but 25% are a result of germline genetic mutations. Hereditary cases occur either in isolation (familial medullary thyroid carcinoma [FMTC]) or as part of MEN syndrome type 2 (MEN2A or MEN2B).[126,191
Диагноз
Although any thyroid nodule could potentially harbor MTC, historical features that may alert the physician to the potential for MTC include a family history of MTC, PCC, hyperparathyroidism, or other manifestations of MEN2 syndromes.[9] The median age of diagnosis is approximately 50 years.[192] In a SEER study that included 1252 patients between 1973 and 2002, the authors found that 87% of the cohort were white and 60% were female.[192] As in evaluating all thyroid nodules, neck ultrasound examination and FNA play a major role in diagnosing MTC. In one retrospective study, ultrasound was found to be falsely negative for ipsilateral and central node involvement in 17% and 14%, respectively, of patients with MTC with no ultrasound pathognomonic features for MTC.[193] Hereditary cases are often detected through genetic screening to identify germline mutations in the RET gene. Almost all sporadic cases manifest with a palpable neck mass, which could be either the thyroid mass or a metastatic lymph node. In contrast to sporadic disease, hereditary disease usually manifests as multicentric and bilateral and involves the upper part of the thyroid.[194,195] However, bilateral disease in sporadic MTC with negative RET mutation can occur in up to 9% of patients.[196–198] Lymph node metastases occur in 35% to 50% of patients at initial diagnosis.[199] Therefore, ultrasound evaluation of the central and lateral neck compartments for suspicious lymph nodes becomes a crucial component to the initial diagnosis.[200]
Because the parafollicular C cells are concentrated in the upper, posterior portion of each thyroid lobe, many MTCs arise in a posterior location, causing symptoms such as hoarseness or dysphagia as a result of compression of local structures. If there is any concern for vocal cord function, then direct laryngoscopy should be performed preoperatively.[199] Markedly elevated calcitonin levels can cause symptoms such as flushing, diarrhea, and weight loss.[201] Distant metastasis to the liver is the most frequent site.[202] CT scan is a very sensitive imaging modality for detection of local nodal and distant lung metastases. Liver metastases are best detected with MRI, and routine use of fluorine-18 fluorodeoxyglucose (18F-FDG) PET-CT is not recommended in MTC evaluation because it is less sensitive.[190,203]
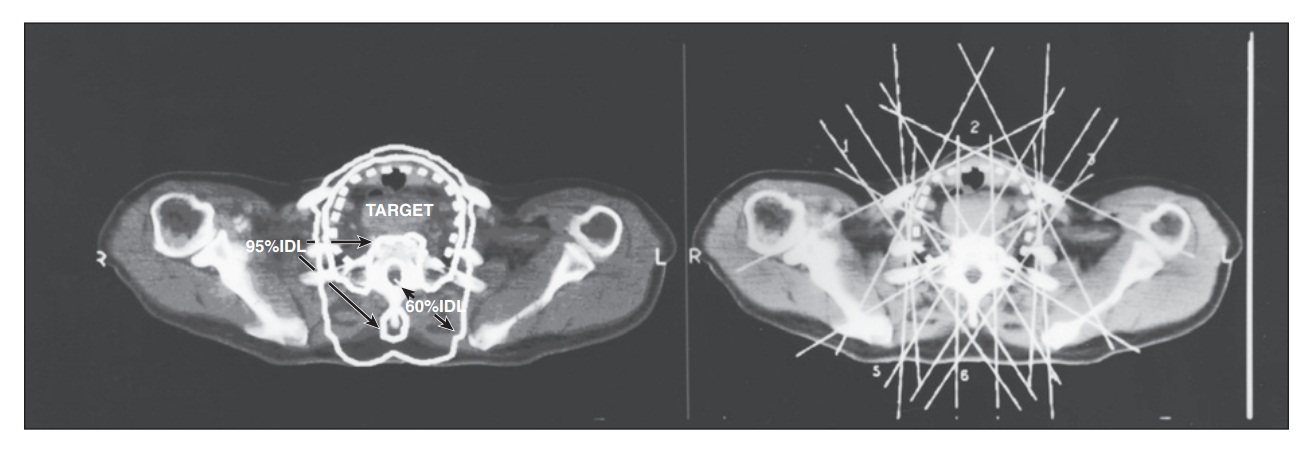
Фигура 68.6. External beam radiation for thyroid carcinoma can be quite complex. On the left, the target for this bulky thyroid carcinoma is marked with a dashed line. The high-dose volume (95% dose line) encompasses this target while avoiding the spinal cord. The radiosensitive spinal cord is in the 60% isodose line, which permits delivery of doses up to 70 Gy with this plan. On the right is a superimposition of the six cross-firing fields that are used to create this dose distribution. The fields either avoid the spinal cord or include a lead block to shadow the spinal cord, protecting it from the high-dose radiation. The treatment planning and dosimetry of thyroid carcinoma treatment is one of the most complex challenges in radiation oncology.
FNA sensitivity in detecting MTC ranges from 50% to 80% and can be improved with the addition of immunohistochemical staining of calcitonin.[204,205] A meta-analysis of 15 publications showed an accuracy of FNA of less than 50%.[206] FNA characteristics of MTC include the presence of stromal amyloid without thyroid follicles. Because spindle-shaped cells may be seen, MTC can be mistaken for parathyroid carcinoma or ATC unless the specimen is stained for calcitonin, chromogranin A (CgA), or carcinoembryonic antigen (CEA)—substances produced by MTC that confirm the diagnosis. A more sensitive technique than immunohistochemistry on cytology specimens is to measure the calcitonin level in the washout fluid from an FNA.[207] In addition, the presence of calcitonin messenger RNA (mRNA) has been performed when the cytologic or histologic diagnosis remains unclear.[208]
Several serum markers can confirm the diagnosis of MTC and are useful in following patients for recurrence and metastases. Calcitonin is commonly elevated in patients with MTC. Although a small percentage of normal patients will have some elevation in calcitonin, patients with a diagnosis of MTC typically exhibit levels above 100 pg/mL. In borderline cases, the diagnosis can be clarified by stimulating the calcitonin with either intravenous calcium gluconate or pentagastrin. Of note, calcitonin secreted by nonthyroid tissue such as lung cancer or prostate cancer does not elevate with calcium gluconate or pentagastrin stimulation. Currently available immunochemiluminometric assays (ICMAs) are more sensitive and specific for monometric calcitonin and largely eliminate cross-reactivity with procalcitonin.[190]
Before the advent of genetic testing, these stimulated measurements were used to screen patients at high risk for MTC.[209] The degree of calcitonin elevation correlates with tumor burden, with nodal metastases found with basal calcitonin levels of 10 to 40 pg/mL and distant metastases found with calcitonin levels greater than 150 pg/mL. Patients with calcitonin levels greater than 3000 pg/ mL are likely to have widely metastatic disease and are unlikely to experience a cure.[210]
Preoperative measurement of serum CEA can also help risk-stratify patients. Overall, CEA elevations occur in more than 50% of patients with MTC, but a preoperative serum CEA level greater than 30 ng/mL highly predicts the inability to cure the patient with surgery.[211] CEA levels above 100 ng/mL may signify extensive lymph node and distant metastases. Following CEA levels postoperatively can also be used to monitor disease progression. Simultaneous increases in CEA level and calcitonin level indicate disease progression,190] whereas elevation of CEA level in the presence of a sТаблица calcitonin level is associated with a worse prognosis because it may indicate tumor dedifferentiation and distant metastases. However, normal CEA or calcitonin level is rare and could represent an advanced dedifferentiated MTC or misdiagnosis.[190] Other markers, such as CgA and serotonin, may be elevated in patients with MTC, as with many other NETs, but calcitonin and CEA are the most useful for following MTC patients in the long term.[192,199,212]
Genetic testing plays an important part in the initial management because it can be used to identify familial disease and to risk-stratify patients. Germline mutations in the RET gene characterize familial disease.[213] A small percentage of apparently “sporadic” disease will also carry germline RET mutations, but truly sporadic cases frequently harbor somatic RET mutations. For sporadic MTCs lacking RET mutations, 18% to 80% have been found to have HRAS, KRAS, or NRAS mutations.[214–216] In sporadic MTC, presence of RET, especially RET codon M918T, mutation usually predicts a poor prognosis.[190,217,218]
Commercial testing is performed through polymerase chain reaction (PCR) amplification of the patient’s germline DNA obtained from the patient’s white blood cells. A spectrum of tumor aggressiveness exists among the various RET mutations, and the timing of prophylactic thyroidectomy is based on the specific mutation. Once a patient tests positive for a germline RET mutation, the patient should be carefully counseled regarding the risk to other family members and the patient’s children. At-risk family members should be identified and also tested so that prophylactic thyroidectomy can be offered at the appropriate time. Although some overlap exists for genetic mutations associated with MEN2A and familial MTC, distinct mutations are usually associated with MEN2B.[219,220]
Лечение
Complete surgical excision is the treatment of choice for MTC. The minimum extent of surgery for patients with clinically apparent disease is a total thyroidectomy with bilateral central neck dissection. Eighty-one percent of patients with palpable disease have central neck lymph node metastases, and the addition of central neck dissection improves cure rates over total thyroidectomy alone in patients with clinically evident disease at presentation.[221,222] The involvement of ipsilateral and/or contralateral nodes has been found to be positively correlated with presence and number of central nodes metastasis.[223] Furthermore, calcitonin level is helpful in preoperative risk assessment of nodal disease. No risk of lymph node metastasis was found when serum calcitonin level was less 20 pg/mL in a study of 300 patients with MTC.[224] In this study, metastasis to the ipsilateral central neck, ipsilateral lateral neck, contralateral central neck, contralateral lateral neck, and upper mediastinum were associated with calcitonin levels greater than 20, 50, 200, and 500 pg/mL, respectively.[224] The initial approach to lateral neck lymph nodes continues to evolve. Historically, the initial surgical treatment included an ipsilateral lateral compartment neck dissection because up to 80% of patients will have ipsilateral nodal metastases.[196] However, current guidelines recommend performing an ipsilateral lateral neck dissection if ultrasound or physical examination detects lymphadenopathy in the lateral neck, if central compartment lymph nodes are involved, or if the primary tumor is greater than 1 cm.[200] Intraoperative assessment of nodal disease by surgeons has sensitivity of 64% and specificity of 75%.[221] Contralateral lateral neck dissection is added when patients have bilateral tumors or there is extensive lymph node disease on the ipsilateral side. Because some patients require extensive neck dissection, these procedures are often staged.[199,200] Unlike DTC, in which micrometastatic disease can be effectively treated with RAI ablation, the only effective treatment for MTC is complete surgical resection. Therefore all evident disease must be resected for the best chance of long-term cure. According to the revised ATA guidelines, complete thyroidectomy is not indicated unless the patient has a RET germline mutation, elevation of serum calcitonin level, or evidence of residual MTC on images.[190]
Prophylactic thyroidectomy is recommended for at-risk family members in hereditary MTC. Current recommendations for the timing of prophylactic thyroidectomy balance the need to remove the at-risk organ before it develops clinically apparent disease with the risks of surgery. In hereditary MTC, an age-related progression exists from C-cell hyperplasia to carcinoma, and, ultimately, nodal metastases. The optimal timing of prophylactic thyroidectomy depends on the risk level of the RET mutation. In general, current guidelines recommend operating on children with MEN2A and familial MTC by age 5 years, whereas those with MEN2B should be operated on before 6 months of age.[225] Prophylactic surgery should consist of at least a total thyroidectomy. The role of prophylactic lymph node dissection in familial disease remains controversial. Lymph node metastases are present in 6% of screened patients,[226] and therefore some argue that prophylactic central lymph node dissection should be performed. Opponents to this approach state that with normal preoperative ultrasound findings, normal calcitonin level (basal and/or stimulated), and a normal CEA level, the risk of occult nodal disease is very lowand does not outweigh the risks of a central neck dissection such as permanent hypoparathyroidism.[9,221,226] Because any complications resulting from prophylactic surgery become lifelong problems for the patient, experienced surgeons should perform prophylactic surgery. Before proceeding with surgery, the surgeon should screen patients with hereditary disease for associated conditions such as PCC (MEN2A and MEN2B) and hyperparathyroidism (MEN2A).[220,226]
All patients will require thyroid hormone replacement once the thyroid is removed, but TSH suppression is not required. After surgery, the next phase of treatment is surveillance. This begins 2 to 3 months postoperatively with a new baseline calcitonin and CEA measurement. If the calcitonin is undetectable, these patients can be followed with yearly calcitonin measurements. Imaging is undertaken when the calcitonin level rises.
A spectrum of disease severity exists for both hereditary and sporadic MTC, and therefore the natural history of MTC varies widely. Distant metastases in the lung, liver, or bone can arise and lead to death quite quickly. On the other hand, many patients live with a large tumor burden and very high calcitonin levels with few symptoms. Others develop intracТаблица diarrhea. In this case, cytoreductive surgery[227] or somatostatin analogues such as octreotide can palliate severe symptoms.[227] Overall 10-year survival rates have been reported as 75% and 95% for tumor confined to the thyroid and regional stage disease, respectively, in a SEER registry study.[192] In this study, age and stage were found to be stronger predictors of survival in univariate analysis.[192] Furthermore, calcitonin doubling times of less than 6 months indicate worse prognosis, with 5-year and 10-year survival rates of 25% and 8%, respectively.[228] Conventional chemotherapy regimens with doxorubicin, dacarbazine, capecitabine, and 5-fl ouracil (5-FU) have demonstrated limited efficacy in patients with MTC. According to the revised ATA guidelines, systematic chemotherapy should not be used as a first-line treatment.[190] Newer, targeted therapies block the RET receptor tyrosine kinase or its multiple downstream pathways, such as the extracellular signal-related kinase (ERK), phosphatidylinositol 3-kinase (PI3-K)/Akt, p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase pathways.[106,199,229] Some of these tyrosine kinase inhibitors inhibit multiple signaling pathways simultaneously.[106,230] These targeted therapies have been evaluated in multicenter trials.[230,231]
The FDA has approved one of these targeted therapies, vandetanib, for treatment of metastatic MTC. Vandetanib is a small-molecule inhibitor of the VEGF receptor, epidermal growth factor (EGF) receptor, and the RET tyrosine kinase.[232] In a randomized controlled clinical trial, patients treated with vandetanib experienced a median PFS of 22.6 months compared with 16.4 months in patients treated with placebo.[230] Another FDA-approved tyrosine kinase inhibitor is cabozantinib, which targets VEGFR1 and VEGFR2, c-MET, and RET. In a phase I/II trail of 35 patients with MTC, 17 patients had a partial response.[233] Patients on tyrosine kinase inhibitors should be monitored for hypothyroidism.[190] Important to note, none of these agents have been shown to prolong overall survival. More research is currently being conducted on other potential therapies.